Vanessa Yoon Calvelo, PhD
Published: June 6, 2023
The Drug Development Pathway: Overview and Challenges
The four phases in the drug development pathway are:
- Early discovery and development
- Clinical testing and trials
- FDA review
- Post-market research & monitoring
The development process of a new medicine across all therapeutic areas can take anywhere between ~12-15 years. The complexity of drug development has increased in the past few decades, requiring a phase of preclinical development, investigational new drug (IND) application, and complete clinical testing before submission for marketing approval from major regulatory agencies (ie. FDA, EMEA).
In addition to the lengthy process, pharmaceutical companies face challenges in profitability and growth as the number of truly innovative new medicines that are approved by regulatory agencies continues to decrease. The average cost for these companies to bring a new medicine to market is estimated to be ~$1.8 billion, and is rising rapidly. With these exorbitant costs and fewer new medicines reaching the market, the impact on the health and well-being of millions of patients could ultimately be devastating as incidences of diseases continue to rise.
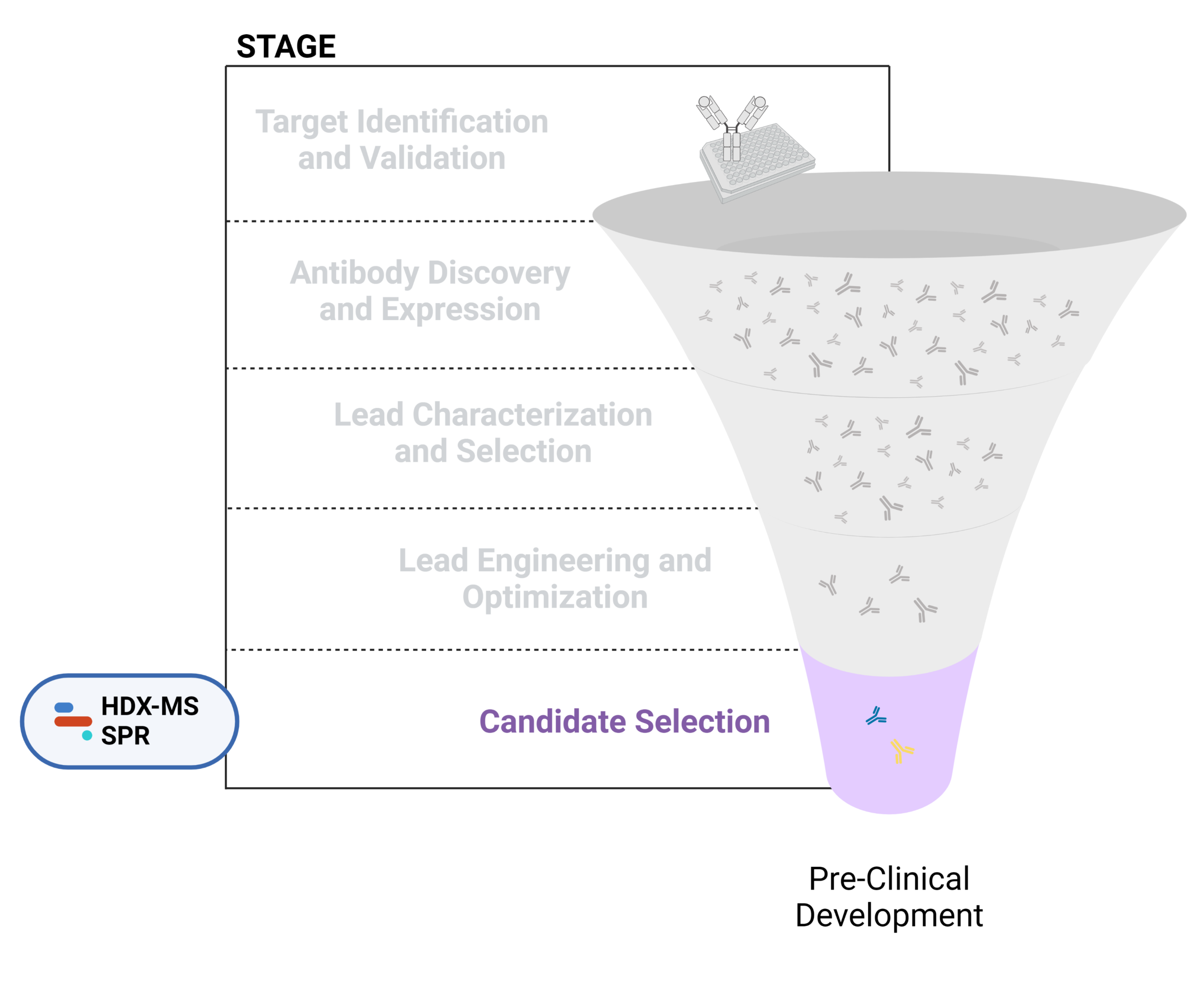
Improving the research and development (R&D) productivity of these drug discovery pipelines often requires streamlining the early discovery and development phase to result in fewer attrition rates of candidates that enter the phase of clinical testing and trials. Below, we dive into the early discovery and development phase and provide a list of our resources available that are associated with each stage involved in discovering therapeutic antibodies (Figure 1).
Early Discovery and Development of Therapeutic Antibodies
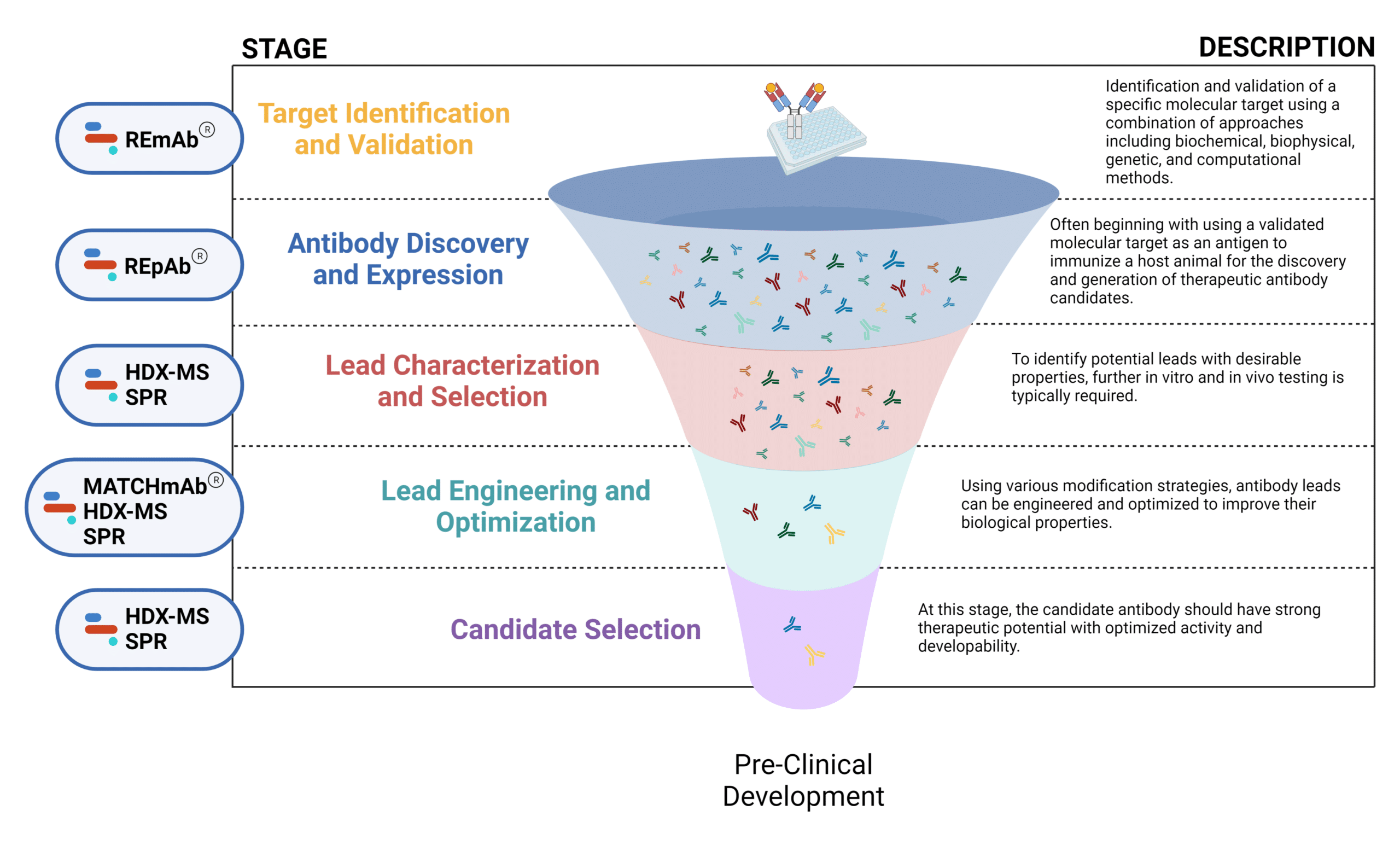
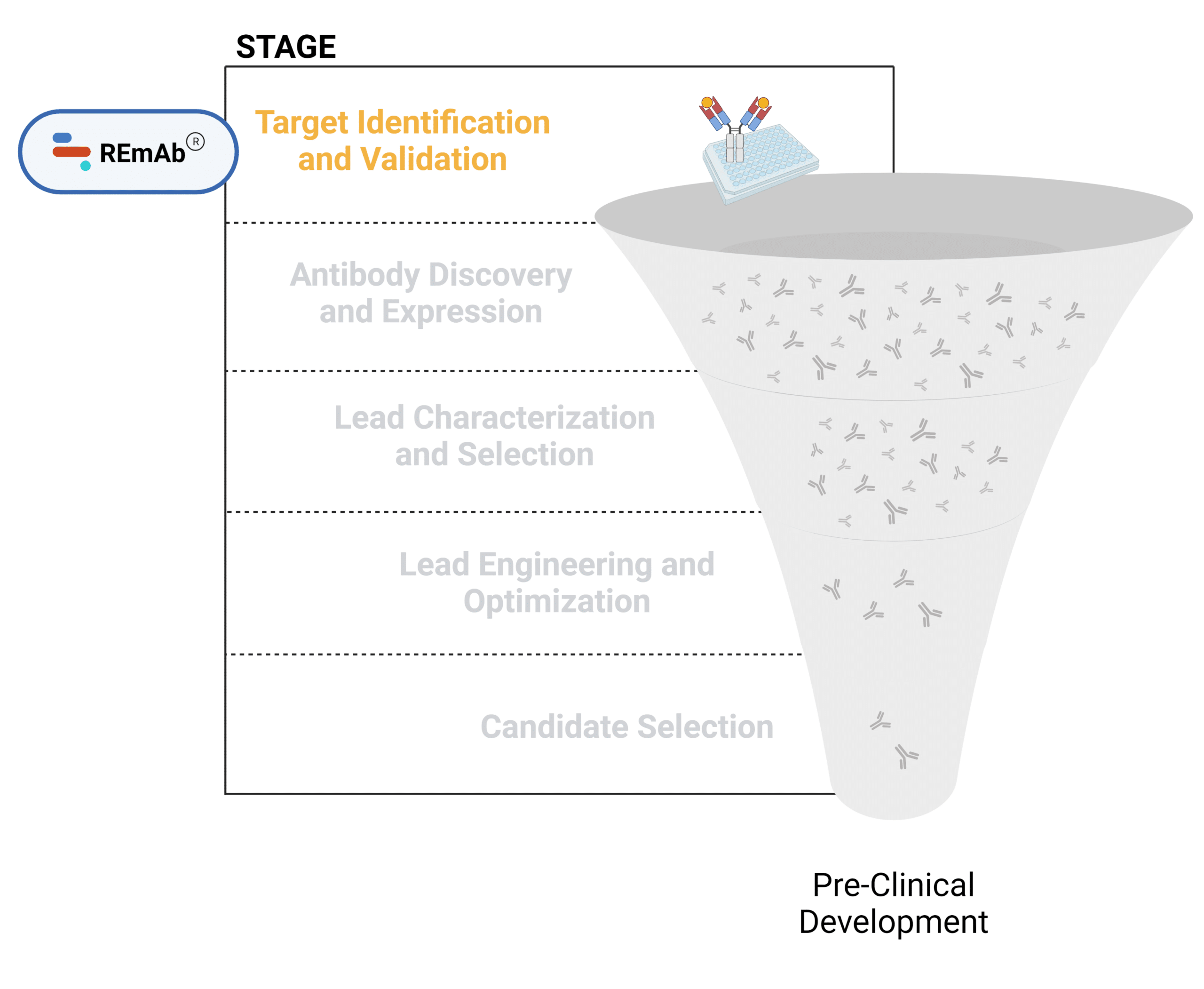
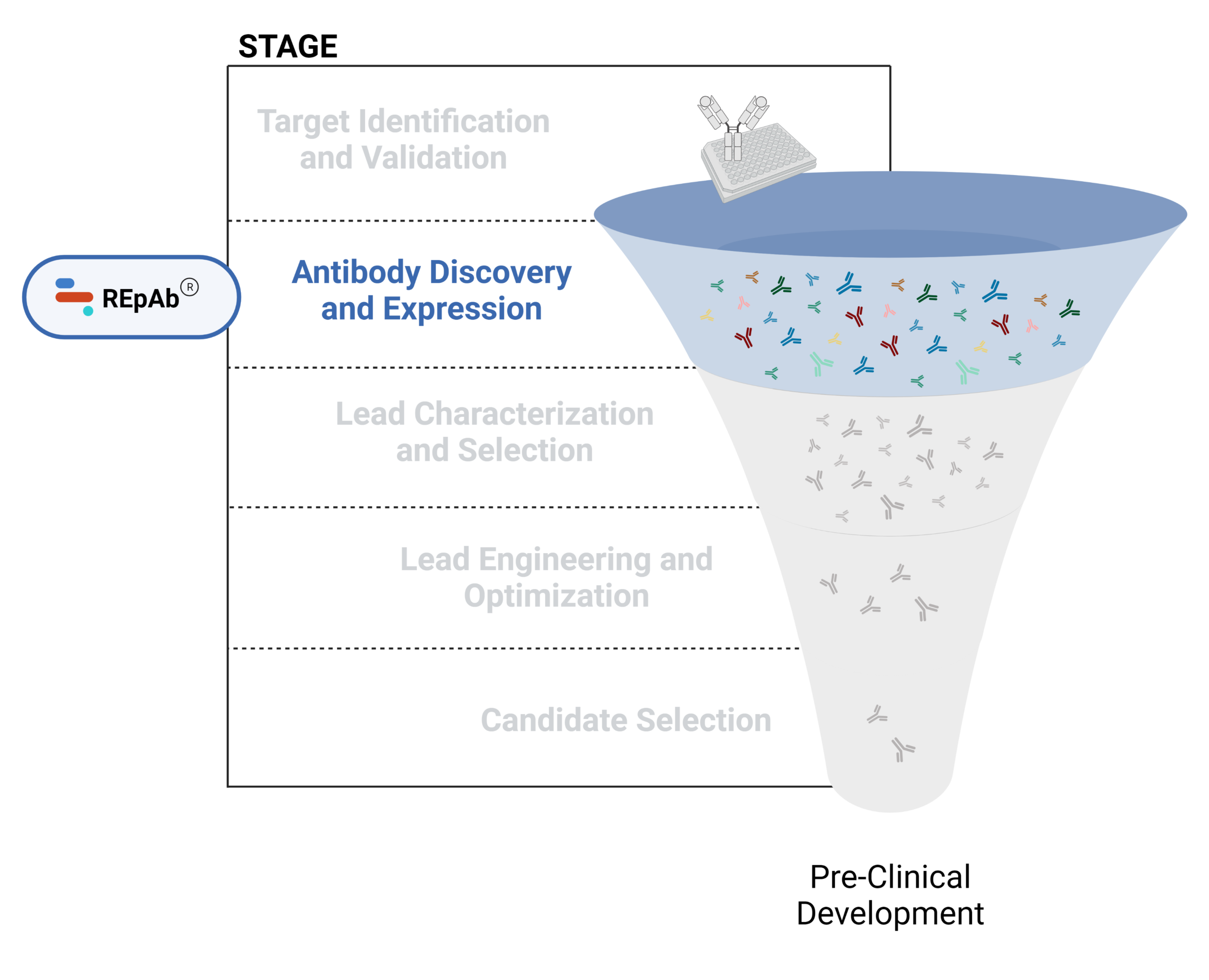
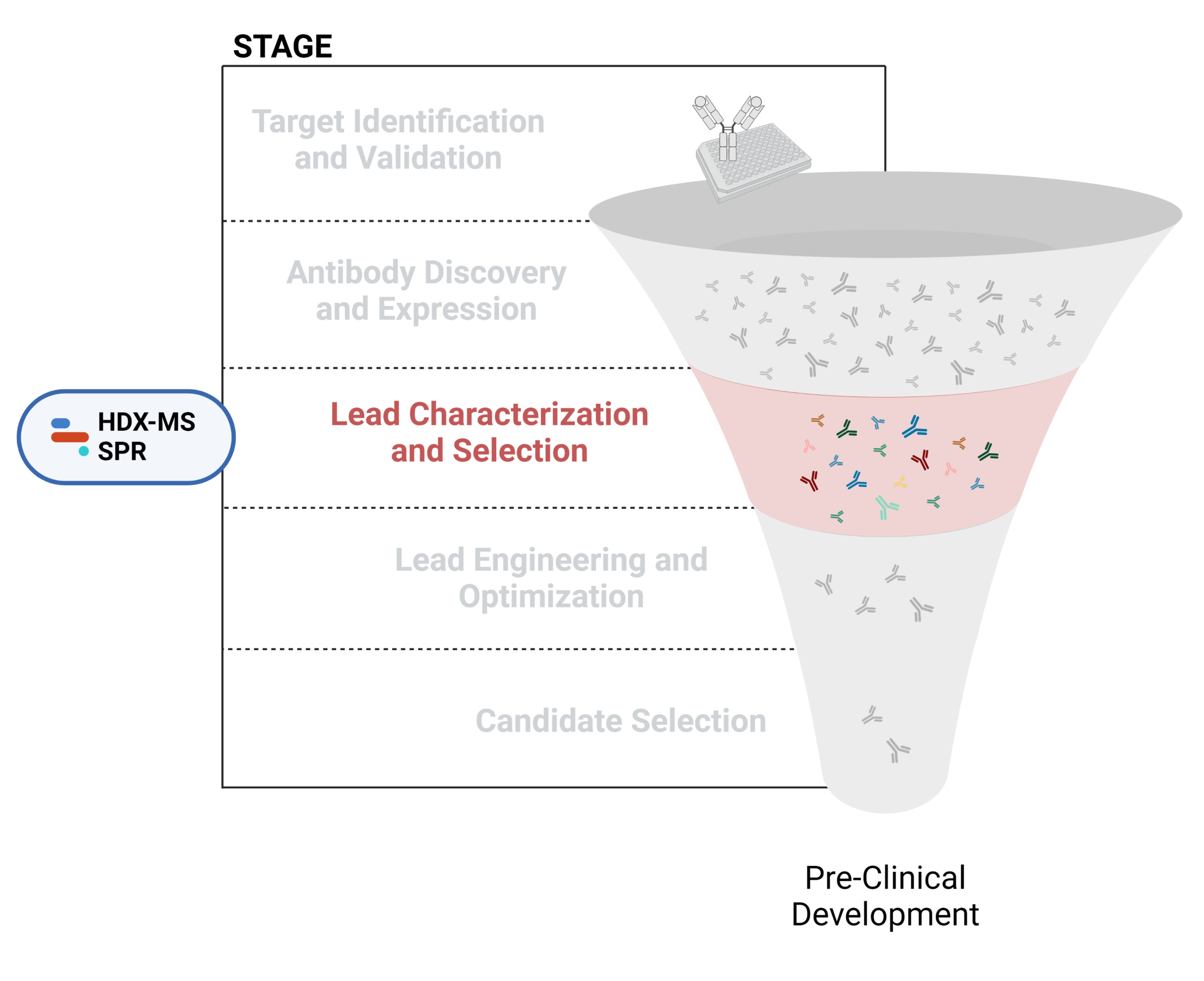
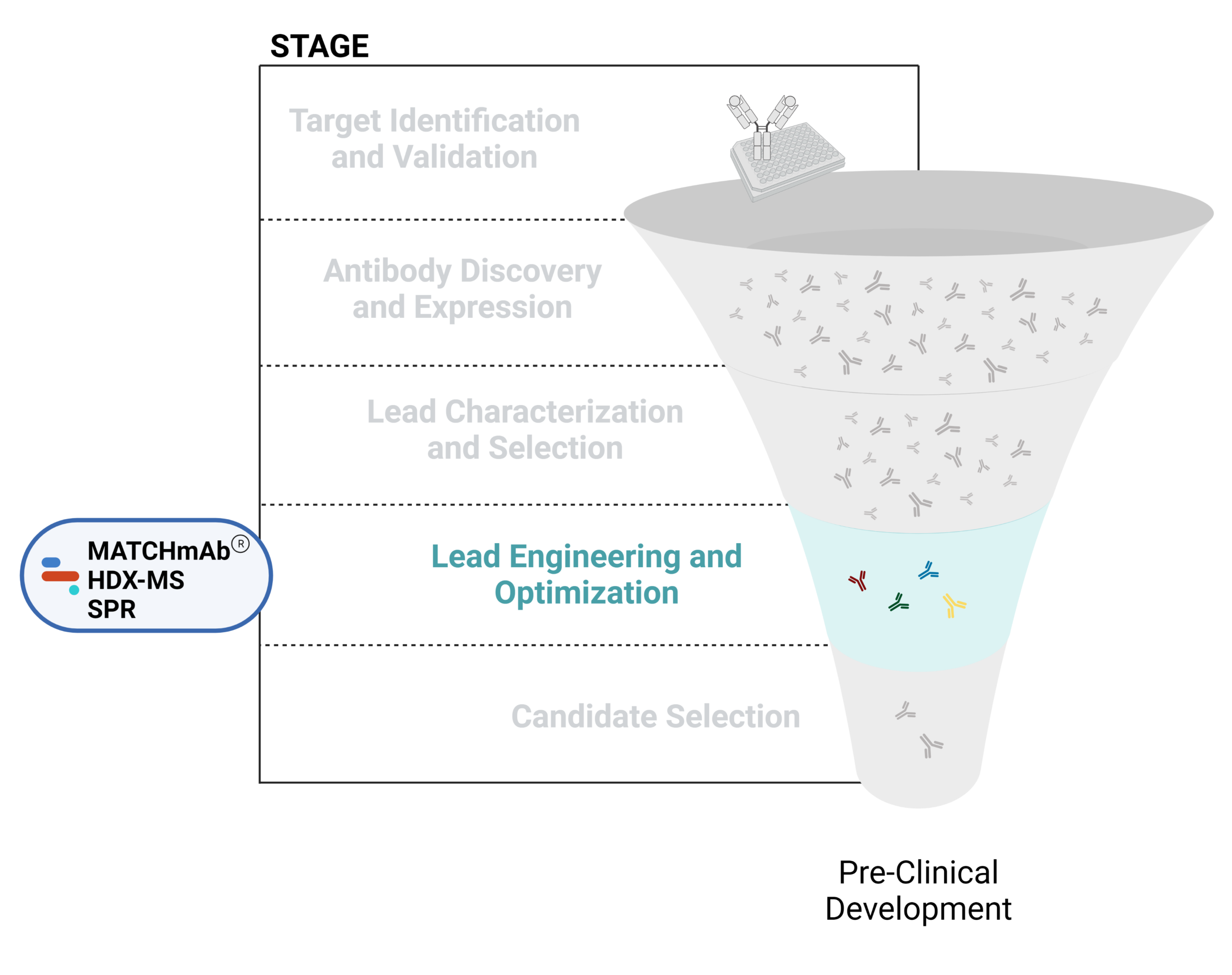
Figure 1. Five stages of the early discovery and development phase in the drug development pathway.
Target Identification and Validation
Identification and validation of a specific molecular target (ie. receptors, antigens) using a combination of approaches including biochemical, biophysical, genetic, and computational methods.
Check out our resources below on next generation protein sequencing and the applications for target identification and validation.
- Understanding and validating tool antibodies and reagents used to study the target: Antibody Validation and its Use Cases
- Understanding competing IP interests about the target: Secure Full Antibody and Target IP Protection with Next Generation Protein Sequencing
- Protein Structure and How to Study It
- Next Generation Protein Sequencing in Veterinary Medicine and Industry
Antibody Discovery and Expression
The discovery of antibodies involves a combination of in vitro and in vivo methods often beginning with using a validated molecular target as an antigen to immunize a host animal for the generation of therapeutic antibody candidates. Common technologies for antibody discovery include hybridoma generation, B cell sequencing, display technologies, and de novo polyclonal antibody (pAb) sequencing.
Learn more about our mass spectrometry-based platform for antibody discovery in our latest articles below.
Lead Characterization and Selection
To identify potential leads with desirable properties, further in vitro and in vivo testing is typically required. Characterization of structural, physicochemical, immunochemical, and functional properties can contribute to the evaluation and selection of ideal lead candidates for downstream development.
Please refer to our latest resources below for further information on our antibody characterization offerings.
Lead Engineering and Optimization
Using various modification strategies, antibody leads can be engineered and optimized to improve their biological properties including effectiveness, affinity, and immunogenicity. Engineering efforts have focused on expanding the applications of antibody therapeutics by generating humanized antibodies, antibody fragments, multispecifics, and antibody-drug conjugates.
Check out our articles below to learn more about the applications of proteomics-based technologies in antibody engineering and optimization efforts.
Candidate Selection
Once the antibody leads have undergone the four stages of early discovery and development mentioned above, these antibodies are either selected or not selected for further rigorous characterization. As the final one or two candidates, these antibodies should have strong therapeutic potential with optimized activity and developability, which are chosen for pre-clinical development.
For a more detailed discussion on the therapeutic antibody discovery process, please refer to our article: Therapeutic Antibody Discovery: From Target to Candidate.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics