 Written by:
Written by:
Genya Gorshtein, MSc
Published: November 1, 2022
Introduction
Antibody engineering encompasses various development, production strategies, and modification techniques to improve the biological properties of monoclonal antibodies (mAbs) as therapeutic agents. During the development stages of antibody therapeutics, engineering methods are typically applied following antibody discovery, screening, and selection efforts (Figure 1). Some of the essential properties sought after in the development of therapeutic mAbs include: low immunogenicity, high-affinity antigen binding, and high stability. As a result, many of the objectives in antibody engineering involve:
- Reducing immunogenicity through efforts in chimerization, humanization, or species backbone switching of mAbs
- Performance optimization through in vitro affinity maturation or generation of bivalent formats
- Increasing half-life, reducing or optimizing effector functions through Fc engineering or Fc silencing
- Targeting more than one receptor through efforts in engineering bispecific or multispecific antibodies
- Simplifying production or increasing biological activity through generation of antibody derivatives, such as single-chain variable fragments (scFvs) or fragment antigen-binding (Fab) fragments
- Conjugation of chemical payloads for the generation of antibody-drug conjugates (ADCs)
- Assay development by labeling antibodies with biotin, HRP, or fluorophores
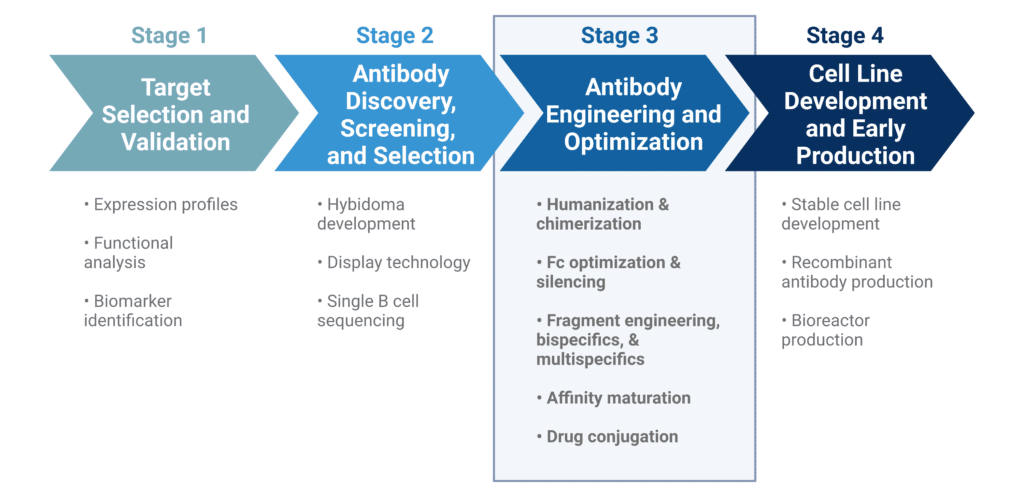
Figure 1. Stages of antibody therapeutic development.
Approaches for Engineering Antibody Therapeutics
Developments in antibody engineering technologies have led to more complex and fine-tuned structures derived from the basic antibody molecule – from scFv or Fab fragments to humanized, chimerized, or bispecific antibodies. Some of the common approaches to antibody engineering will be discussed below.
Chimerization and Humanization
Murine-derived antibodies cause immunological responses in patients, thereby causing rapid elimination of these molecules and significantly limits their therapeutic potential. This response produces human anti-mouse antibodies (HAMA), which recognize the therapeutic mAb as a foreign molecule. This is often due to differences in glycosylation patterns and other post-translational modifications between human and mouse antibodies.
Consequently, engineering efforts have focused on the development of chimeric and humanized antibodies to alleviate immunogenic responses. Chimeric antibodies are engineered where murine constant regions were replaced by human constant regions, leaving mouse-derived variable regions to interact and bind the antigen (Figure 2). Engineering humanized antibodies involves complementarity determining region (CDR) grafting, a process that incorporates mouse-derived CDRs into human-derived framework and constant regions.
Fully human antibodies utilize fully human antibody sequences, selected from isolated human peripheral blood mononuclear cells (PBMCs) or transgenic mouse models. These engineered antibodies are associated with a lower risk of inducing an immunogenic response, thus improving their therapeutic potential.
Antibody Fragment and Bispecific Engineering
In some cases, engineering of antibodies into their minimal binding fragments is advantageous over their full-length formats. Their smaller size allows them to penetrate through tissues, reach embedded epitopes of the antigen, and are sometimes easier to produce at a large scale. Variable heavy chains and light chains can be isolated and amplified from any candidate antibody and engineered into various fragmented formats (Figure 2). Many forms of fragmented antibodies have been engineered for therapeutic purposes, including:
- Fv fragment – composed of only the variable region from either the heavy or the light chain
- VHH or nanobody – composed of the antigen binding fragment of the heavy chain only
- Fab fragment – composed of the variable domain, first constant region of the heavy chain and the light chain
- ScFv – composed of the variable domain from both the heavy and the light chain, connected through a linker rather than the Fc region
- Diabody or bispecific fragments – composed of two different antigen binding domains, providing dual specificity
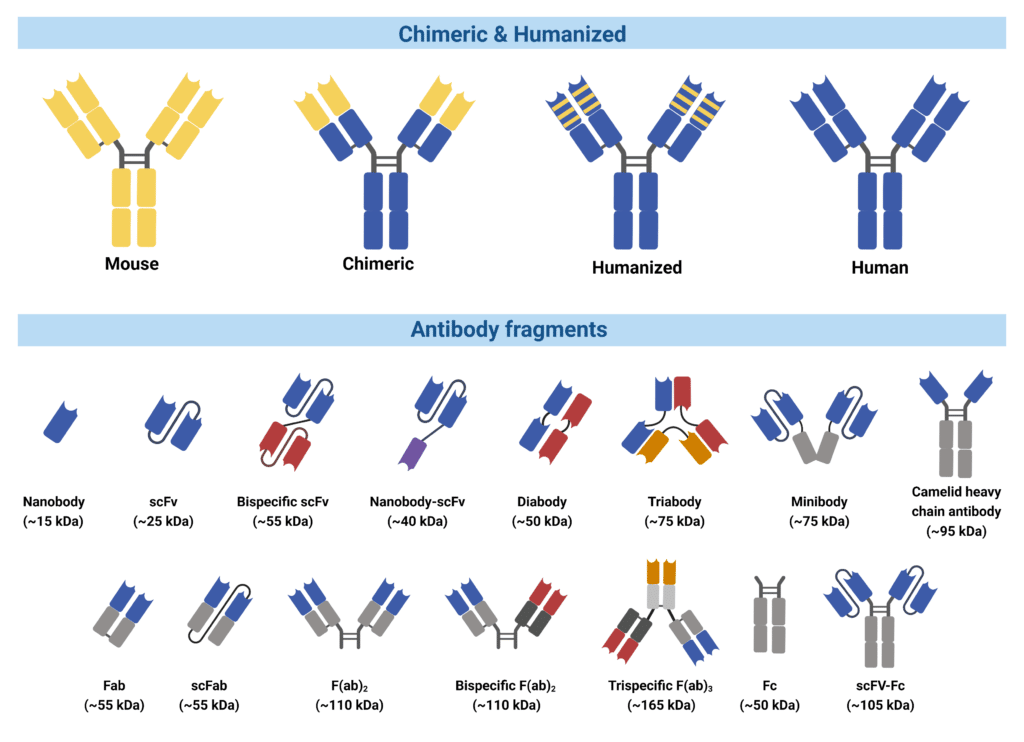
Figure 2. Types of engineered antibodies and antibody fragments
In Vitro Affinity Maturation
Similar to in vivo somatic hypermutation, in vitro affinity maturation introduces sequence diversity to produce a library of mutational variants. Display technology, such as phage, yeast or ribosomal methods are used to create and enhance high-affinity binders. Affinity maturation may sometimes be combined with antibody humanization, as loss of affinity may occur as a result of CDR grafting. Thermodynamic analysis through surface plasmon resonance (SPR) can further characterize antibody candidates to identify high affinity binders.
Fc Engineering
The Fc domain of an antibody mediates the activation of effector functions of the immune system. Complement dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and antibody-dependent cellular phagocytosis (ADCP) are activated through receptor binding to the Fc fragment and induce tumour cytotoxicity and cell death.
Fc engineering involves enhancing the effector function of therapeutic antibodies to improve their potency and therapeutic potential. Improving binding affinity and capacity of the Fc domain for their cognate Fc receptors can improve the therapeutic efficiency. Conversely, reduced effector functions may be advantageous for receptor blocking, and thus requires Fc engineering to silence effector functions.
Conjugation and Labeling
Antibody sequences can be engineered to make them more amenable to conjugation, depending on the linker technology used. The drug-to-antibody ratio (DAR) is an important attribute of ADCs; impacting drug efficacy, potency, and pharmacokinetic properties. Modulation of the antibody sequence can direct site-specific disulfide conjugation to achieve uniform drug stoichiometry and homogenous ADC populations. ADC design for site-specific conjugation may involve engineering reactive cysteine residues at defined sites, replacing cysteines with serine residues, and incorporating novel amino acids into the antibody molecule.
Labeled antibodies are an essential research tool used to detect and quantify antigens in a variety of immunoassays, such as Western blots, flow cytometry, and ELISAs. Labels such as biotin, horseradish peroxidase (HRP), or fluorophores can be linked to antibodies through charged amino acids, tyrosine residues, carbohydrates or sulfhydryl groups.
Driving Antibody Engineering with Next Generation Protein Sequencing and Proteomics
The emergence of antibody engineering has expanded the use and diversity of antibody-based therapies. Engineered antibodies may experience increased complexity; as such they may benefit from in-depth characterization to ensure their reproducibility, specificity, and affinity. At Rapid Novor, we support antibody engineering efforts in all stages of development with:
De novo antibody sequencing – Obtaining the amino acid sequence of antibodies known to perform well against a target can help researchers to understand how they work, and guide the discovery and/or engineering of novel antibody constructs: species switching, humanization, fragment design, affinity maturation, and in silico design. Rapid Novor utilizes a well-established mass spectrometry-based service to sequence antibodies directly from the mass spectral data, without reference to genomic sequence information..
SPR kinetic binding analysis – SPR kinetic binding analysis will reveal properties such as specificity, affinity, and efficiency of antibody-antigen interactions to help evaluate engineering strategies and select therapeutic candidates. SPR can characterize diverse biomolecules and their interactions, read about SPR kinetic binding analysis to learn more about its applications.
HDX-MS epitope mapping – HDX-MS epitope mapping can characterize antibody-antigen protein complexes, to accurately determine linear, conformational, and structural epitopes. Read about HDX-MS epitope mapping as a powerful technique for antibody characterization.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

