 Written by:
Written by:
Genya Gorshtein, MSc
Published: September 14, 2022
Introduction
An antibody-drug conjugate (ADC) is a monoclonal antibody (mAb) with a covalently attached cytotoxic payload through a chemical linker. Traditional chemotherapies lack tissue specificity, leading to severe side effects caused by toxicity to healthy cells. The mAb component of the ADC provides specificity to the target tissue, directing the small-molecule cytotoxic drug to the desired location and reducing off-target tissue effects. The ADC binds the target antigen on the surface of the tumour cell, resulting in its internalization. The cytotoxin is cleaved intracellularly causing DNA or microtubule disruption, leading to cell death through apoptosis. There are currently over 100 ADC candidates in clinical trial stages, with 12 approvals for both hematological and solid tumour cancers. ADCs show potential for non-oncological use as well, such as for autoimmune and infectious disease; however, here we focus on their application as anti-cancer therapeutics.
ADCs as Novel Anti-Cancer Chemotherapeutics
With the combined advantage of high target specificity and potent cytotoxic effects, ADCs are a promising class of anticancer therapeutics. ADCs are administered intravenously and circulate through blood plasma. Specificity and affinity of the antibody to a cancer-specific, or a cancer-associated antigen directs ADCs to target tumour cells. The mAb of the ADC binds its target antigen, typically a cell-surface protein, and the antigen-ADC complex is endocytosed (Figure 1). In subcellular compartments such as the endosome or lysosome, the cytotoxic payload is released from its linker and induces cell death via apoptosis. If the cytotoxin is cell permeable, it may induce a ‘bystander effect’ where it targets neighbouring cancer cells in the tumour microenvironment. The Fc segment may bind Fc receptors of natural killer (NK) cells and macrophages, inducing antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP).
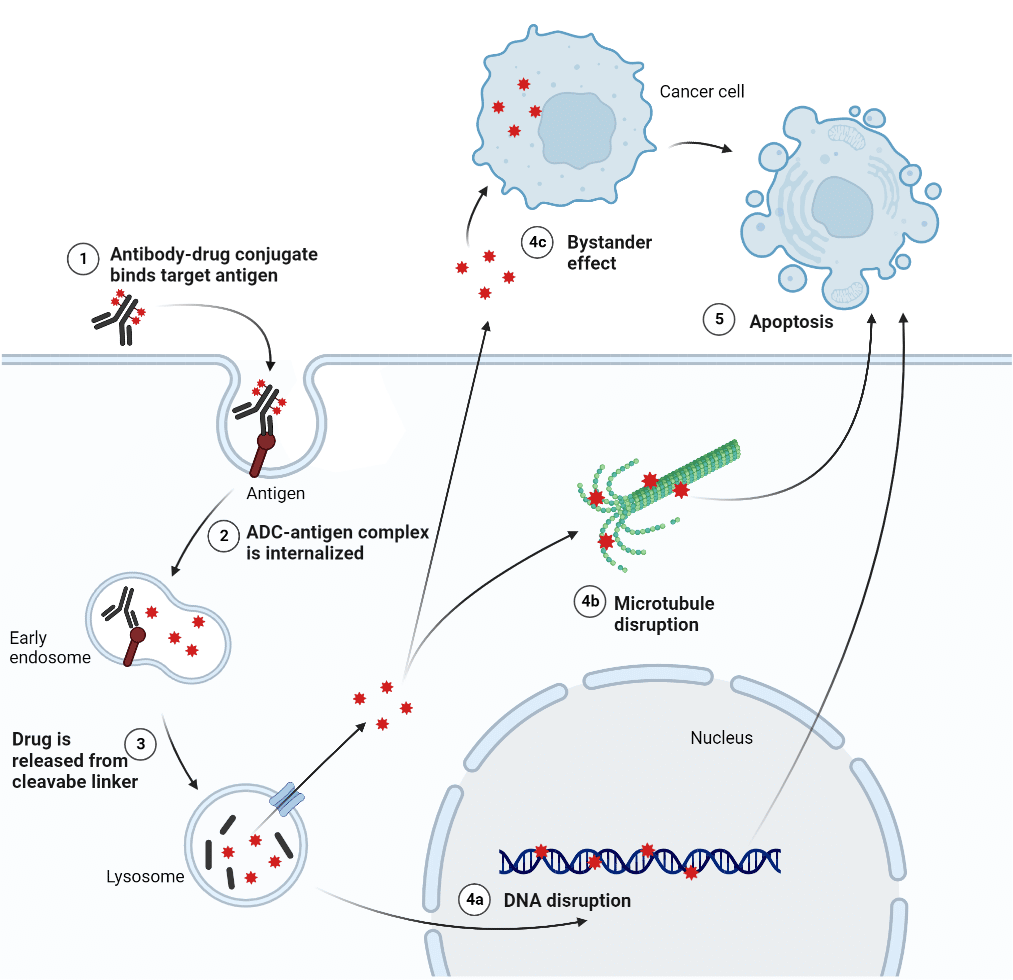
Figure 1. General method of action of ADCs.
Key Components of ADCs
ADCs are composed of a mAb, cytotoxic payload, and a chemical linker (Figure 2). Each component of the ADC influences the efficacy and safety of the therapeutic. Various considerations are made during the development of an ADC candidate including physiological stability, mAb half life and affinity, and drug potency.
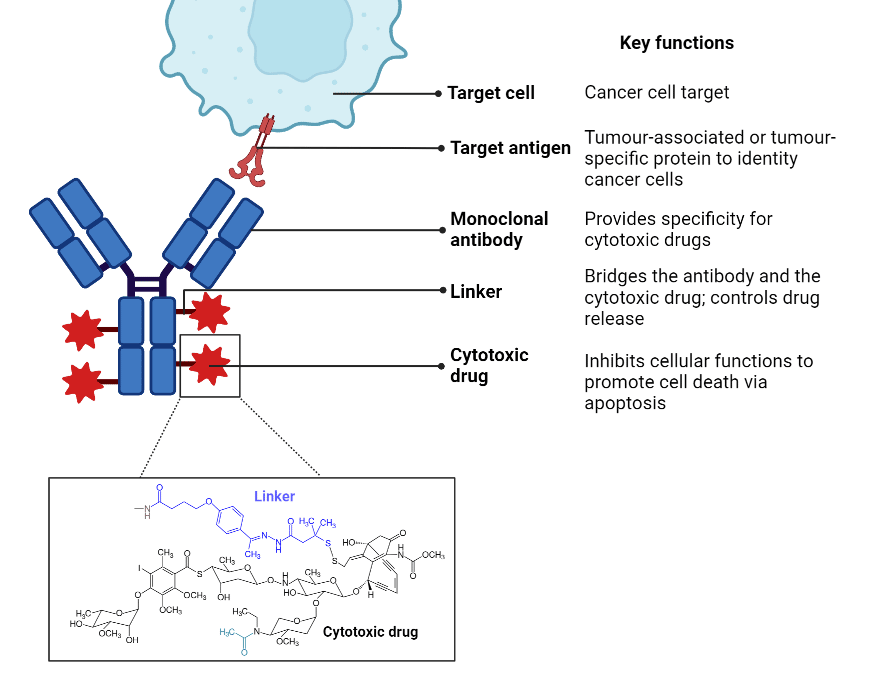
Figure 2. Overview of key components of ADCs.
Target Antigen Selection
The mAb’s affinity for its target antigen facilitates the delivery of the cytotoxic payload to the tumour cells. The target antigen may be a tumour-specific antigen expressed only in cancer, or a tumour-associated antigen with elevated levels in cancer cells and lower levels in healthy cells. To be recognized by circulating ADCs, antigens should be expressed at the cell surface as opposed to a soluble intracellular antigen. Cell surface proteins are regularly internalized via endocytosis; this process allows for the ADC-antigen complex to enter the cancer cells and release the cytotoxic payload. Human epidermal growth factor receptor 2 (HER2) is a common antigenic target of ADCs due to its 100-fold overexpression on the surface of tumour cells and its efficient internalization. ADCs may also target the extracellular matrix and stroma of the tumour microenvironment and pose an alternative to targeting cell surface proteins.
Antibody Moiety
The class of the antibody is an important consideration in engineering the ideal ADC. The antibody class can impact effector functions and the plasma half-life of ADCs. Immunoglobulin G (IgG), specifically the first subtype IgG1, is most used for ADCs due to their ability to elicit strong effector functions such as ADCC, CDC, and ADCP. IgG1 has a longer half-life, which allows for increased exposure of the ADC to target tissue.
Immunogenicity to ADCs can result in unwanted side effects and can significantly impact drug half-life and efficacy. Replacement of mouse-derived antibodies with humanized antibodies reduces the formation of human anti-mouse antibodies and improves the therapeutic potential of ADCs.
Internalization of the antigen-antibody complex facilitates the delivery of the cytotoxin into the tumour cell and is thus a critical factor in ADC efficacy. Efficiency of internalization is dependent on the binding affinity between the antibody and the antigen. Affinity needs to be optimized, as too strong of an affinity may reduce the ability for the ADC to penetrate into the tumour. Binding kinetics needs to be considered as well, since antibodies with a slow off-rate will remain bound longer and could have a stronger cytotoxic effect. Additionally, tumour penetration is affected by the large molecular weight of the antibody (~150kDa) due to the size exclusion through blood capillary and matrix networks. One method to circumvent this issue is by removing the Fc segment of the antibody, thereby reducing its size without influencing antigen specificity and affinity.
Linker
Linkers bridge the antibody to the cytotoxic drug and facilitate its release into the target cell. Cleavable linkers take advantage of the cellular physiological conditions where reduction, hydrolysis, and proteolysis release the cytotoxic payload from the antibody. Release of the payload via hydrolysis is pH-dependent, whereby the ADC is trafficked from a neutral environment (plasma pH 7.0 – 7.5) into an acidic environment (lysosome pH 4.5 – 5.0). Disulfide linkers are sensitive to reduction by glutathione (GSH), which is present in higher intracellular concentrations in cancer cells compared to healthy cells. Enzyme-sensitive linkers are dependent on lysosomal proteases and are commonly used in approved ADC drugs. Lysosomal proteases, such as cathepsin B, are overexpressed in cancer cells and thereby allow for accurate drug release into target cells.
Cytotoxic Payload
Cytotoxic payloads are chosen for their high potency and physiological stability, as approximately 2% of administered ADCs reach their targeted sites. This class of drug includes DNA damaging agents, tubulin inhibitors, and immunomodulators to induce apoptosis in target cancer cells. Tubulin inhibitors interfere with microtubule assembly and cause cell cycle arrest. DNA damaging drugs induce double stranded DNA breaks, DNA intercalation, cross-linking, or alkylation, inducing cell cycle arrest and apoptosis. Immunomodulators, also termed immune-stimulating antibody conjugates (ISACs) are a novel class of ADC drug, which utilize receptor agonists against immune receptors to facilitate anti-tumour immune activity.
Care must be taken in the CMC and analytics process to ensure that the drug-to-antibody ratio is consistent as production scales up. Incomplete conjugation or additional unintended conjugation could have disastrous consequences.
Future Generations of ADCs
New and future generations of ADCs demonstrate increased specificity and cytotoxic potency than early developments. The mAb components of the ADC can be modified to improve penetration into tumours and internalization into cancer cells.
Bispecific ADCs
Bispecific antibodies (bsAb) are monoclonal antibodies that contain two different antigen-binding capabilities. Upon antigen binding, the bsAb acts as a bridge between the two antigens, achieving different effector functions. The use of bsAb in ADCs can improve ADC internalization and improve cytotoxic payload delivery to cancer cells. For example, a bsADC engineered to target HER2 and lysosomal-associated membrane glycoprotein 3 (CD63) improved intracellular trafficking of the ADC to the lysosome. Bispecific ADCs can be used to bind to two different tumour-specific targets as well. These bsADCs can target tumour cells expressing one or both antigens, thereby reducing the potential for cancer cells to escape therapy.
Miniaturized ADCs
Due to the large molecular weight of ADCs, depth of penetration into solid tumours may be limited. Miniaturized ADCs abandon the traditional mAb structure and the cytotoxic payload is conjugated to a single chain variable fragment or polypeptide fragment. PEN-221 is a novel drug candidate that showed improved tumour penetration in small cell lung cancer, by conjugating a microtubule inhibitor cytotoxin to a polypeptide chain targeting somatostatin receptor 2. This reduced the molecular weight of the drug to 2 kDa from the traditional IgG molecular weight of 150 kDa, improving tumour penetration and cytotoxic payload delivery.
De Novo Protein Sequencing Applications in ADC Development
Rapid Novor utilizes a well-established mass spectrometry-based service to sequence antibodies directly from the mass spectral data. De novo protein sequencing can support biologics drug discovery and antibody engineering to further the development of ADC as anticancer therapeutics. Applications can include:
- REmAb® de novo sequencing of monoclonal antibodies to derive and validate sequences of important candidates.
- MATCHmAb® peptide mapping to characterize drug conjugated sites.
- SPR analysis of antibody-antigen interactions to measure key antibody properties (binding affinity, specificity, and kinetics) of lead candidates with optimal therapeutic potential.
- HDX-MS epitope mapping to determine ADC binding sites on target antigens.
- Protein engineering and expression of antibodies and polypeptide fragments for recombinant ADC design.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

