 Written by: Genya Gorshtein, MSc
Written by: Genya Gorshtein, MSc
Published: April 19, 2024
Introduction
The complex nature of diseases often limits the therapeutic efficacy of monovalent antibodies. To address this challenge, a new era of bispecific (bsAbs) and multispecific (msAbs) antibodies has been ushered in by recent advancements in antibody discovery and engineering. These dual- and multi-binding antibody formats simultaneously target multiple disease-related pathways or molecules, offering a promising avenue for enhanced therapeutic outcomes.
In this review, we will discuss the diverse formats of bispecifics and multispecifics, highlighting the engineering approaches underpinning their development, and elucidating their mechanisms of action.
Bispecific and Multispecific Antibody Formats
There are hundreds of different formats of bsAbs and msAbs. As antibodies are composed of individual chains with distinct regions, they possess the potential to function as interchangeable building blocks, leading to an expansive diversity of formats.
These antibodies can broadly be categorized into IgG-like and non-IgG-like formats, with the key distinguishing factor being the presence or absence of the Fc-domain.
IgG-Like Formats
IgG-like bsAbs and msAbs retain their Fc-mediated effector functions, including antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and antibody-dependent cellular phagocytosis (ADCP). Maintaining the Fc-domain extends the serum half-life due to FcRn-mediated recycling, and is generally less prone to immunogenicity concerns due to lack of non-native sequences.
Knob-Into-Hole Design
Traditionally, the production of bsAbs relied on ‘quadroma’ cell lines, which involves the fusion of two distinct hybridomas. However, this method posed challenges due to random pairing of heavy and light chains within the same cell culture. Theoretically, this random pairing could yield 16 different combinations, with only one resulting in a functional bsAb, while the rest remain non-functional or monospecific.
To address this, the ‘knob-into-hole’ design emerged as an early solution (Figure 1). This approach introduces mutations in the CH3 domains, by replacing a small amino acid with a larger one as the ‘knob’ on one heavy chain, and a vise versa as the ‘hole’ on the other heavy chain.
This engineered strategy guides the formation of heterodimeric antibodies over homodimers through electrostatic steering interactions. Additional interchain disulfide bonds have also been engineered into the CH3 domains of heavy chains to further mitigate unwanted mispairing. Despite these advancements, concerns about light chain mispairing persist. Various approaches, including creating bsAbs with common light chains and introducing additional mutations into the VH-VL antibody regions, have been employed to circumvent these challenges.
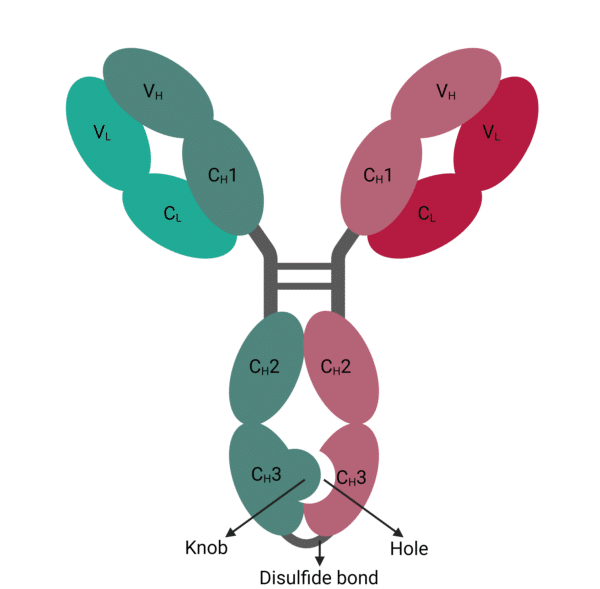
Figure 1. Knob-into-hole bispecific antibody.
Fragment-Based Bispecific Antibody Formats
Fragment-based bsAbs lack the Fc region, and thereby are typically smaller in size resulting in enhanced tissue penetration. However, a drawback to fragment-based bsAbs is their rapid clearance due to lack of FcRn-mediated recycling.
Single Chain Variable Fragment-Based Bispecific Antibodies
ScFv-based bispecifics have become foundational elements for fragment-based bsAbs. bispecifics have emerged as foundational components in fragment-based bsAbs. Their compact structure, composed of two variable domains connected by a flexible linker, demonstrates superior tissue penetration, enhanced epitope accessibility, decreased immunogenicity, and streamlined production methodologies.
ScFvs can be constructed in either VH–VL or VL–VH orientations. Depending on linker length, orientation, and antibody sequence, ScFvs can form dimers, trimers, or tetramers. Various other bsAb formats have been developed based on ScFvs (Figure 2):
- Tandem scFvs: Two ScFvs linked by a flexible peptide linker, resulting in four variable domains connected within a single polypeptide chain. A prevalent bsAb format, bispecific T cell engager (BiTE), is derived from this fragment design.
- Diabody format: Generated by connecting the variable regions of heavy and light chains via a short peptide linker. This linker prevents intrachain pairing, favoring inter-chain pairing and yielding a dimeric complex with two antigen-binding sites.
- Dual affinity retargeting proteins (DART): Similar to the diabody format, but with engineered cysteine residues forming an inter-Fv disulfide bond, resulting in a more rigid and compact structure.
- Tandem diabodies (TandAb): Consisting of two pairs of VL and VH domains connected within a single polypeptide chain, producing a tetravalent tandem diabody.
Single Domain Bispecific Antibodies
Single-domain antibodies are sourced from camelids like camels, llamas, and alpacas, which naturally produce antibodies containing a single variable domain. Known as VHH or nanobodies, these single-domain antibodies can be linked via short peptide linkers with nanobodies of other antigen specificities to generate bispecific nanobodies.
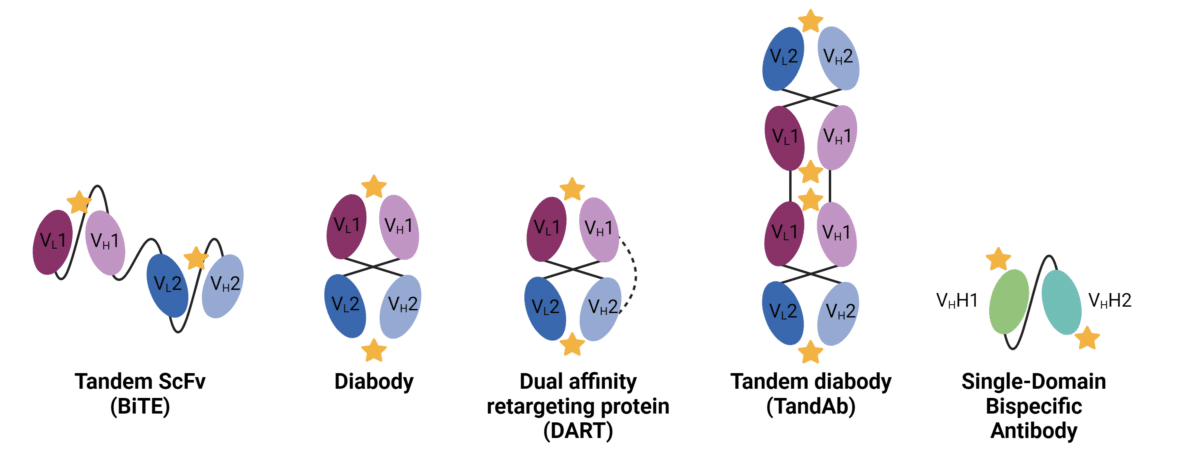
Figure 2. Fragment-based bispecific antibodies. Stars represent antigen binding sites, and dashed lines represent disulfide linkages.
Appended IgG Bispecific Formats
Appended IgGs are a bispecific format in which an antibody fragment is fused to the canonical IgG structure or Fc fragment, either on the N- or C- terminals of heavy chains or light chains. The fused fragments can be variable regions, scFvs, single domain antibodies, or alternative scaffolds, resulting in a wide variety of appended IgG formats (Figure 3). Appended IgGs contain additional linker sequences and non-natural junctions that often require more engineering efforts to promote stability and mitigate immunogenicity.
Dual-Variable Fragment IgGs
Dual-variable fragment IgGs (DVD-IgGs) resemble the standard IgG structure, with appended antigen-binding variable regions (VL and VH) via peptide linkers to the corresponding heavy or light chain. The positioning of the variable domains requires specific optimization to achieve optimal antigen binding. Additionally, DVD-IgGs are bispecific and bivalent for each antigen, meaning they can simultaneously bind to two different antigens. With two binding sites for each antigen, DVD-IgGs possess a total of four binding sites.
Single Chain Variable Fragment IgGs
Single-chain variable fragment (scFv) IgGs are generated by fusing an scFv to any chain or termini of an IgG. This appended bispecific format can create both symmetric and asymmetric configurations, enabling different mechanisms of action.
scFv-IgGs could have two binding sites for each of the two antigens, resulting in a total of four binding sites. In some engineered designs, additional scFv domains could be appended to increase valency beyond bivalency. This could enable enhanced avidity or facilitate the formation of higher-order complexes.
Engineering scFv-IgG bispecifics, three strategies are often employed to maintain high binding affinity and stability of the scFv-IgG:
- Incorporating flexible linkers between the heavy-chain variable and light-chain variable to maintain the stability of the scFv.
- Engineering additional disulfide bonds between the VH and VL domains.
- Selecting optimized and stable scFvs early in the engineering process.
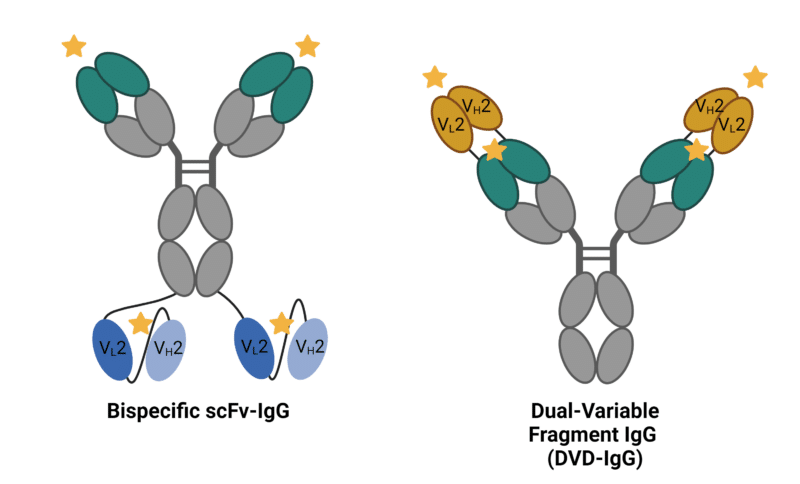
Figure 3. Appended IgG Bispecific Antibody formats. Stars represent antigen binding sites.
Mechanisms of Action of Bispecific Antibodies
The dual- and multi-antigen binding capabilities of bsAbs and msAbs result in more intricate mechanisms of action (MOA) compared to conventional monoclonal antibodies. Typically, the MOA of bsAbs rely on bridging two binding events in close spatial proximity to induce a desired downstream reaction (Figure 4).
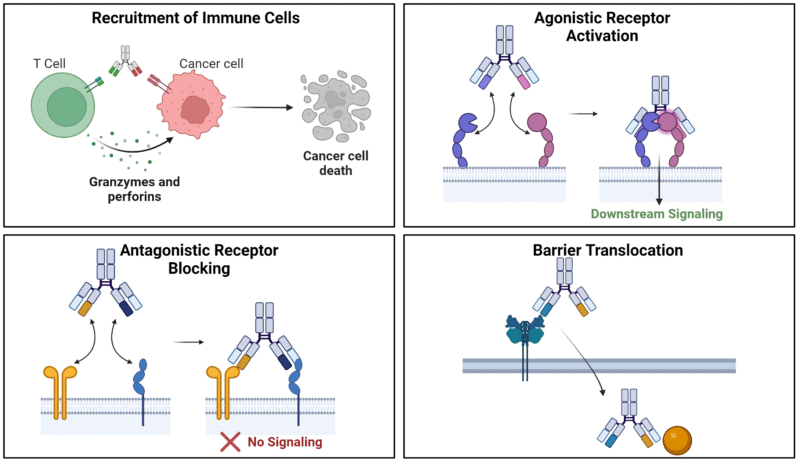
Figure 4. Mechanisms of action of bispecific antibodies.
Recruitment and Activation of Immune Cells
The dual and multi-targeting capabilities of bsAbs and msAbs enable the creation of a physical bridge between two cellular targets. This facilitates the connection of immune cells to tumor cells, directing the immune cell’s cytotoxic effects against the tumor cell.
BiTE antibodies, consisting of two single-chain variable fragments (scFvs) connected by a short linker target a tumor-associated antigen expressed on cancer cells, and the CD3 receptor on T cells. This dual targeting mechanism allows BiTEs to specifically engage both cancer cells and T cells, leading to the formation of an immunological synapse and subsequent T cell activation. Once activated, T cells release cytotoxic molecules such as perforin and granzymes, leading to the death of the cancer cells.
BiTE-mediated T cell activation is unique compared to other bsAbs. BiTEs activate T cell cytotoxicity without costimulation, such as through anti-CD28 antibodies and interleukin-2 (IL-2).
Similar to BiTE antibodies, bispecific natural killer (NK) cell engagers (BiKEs) and tri-specific NK cell engagers (TriKEs) are engineered to simultaneously bind a tumor-associated antigen and an activating receptor on NK cells. BiKEs bind to Fc receptor CD16 (CD16A, FcγRIII), which is an activating receptor in NK cells, resulting in ADCC-triggered tumour cell death. TriKEs have a third scFv which can target another cancer target, or other immune-cell stimulating cytokines to enhance NK cell functions.
Agonistic Receptor Activation
BsAbs can target different antigens on the same cell, leading to the agonistic activation of two cell surface receptors and modulation of signaling pathways. For example, a bsAbs designed as an anti-tumor agonist can activate the IL-2 pathway by binding to both IL-2Rβ and IL-2Rγ subunits. This activation triggers downstream signaling mediated by IL-2, consequently promoting immune cell activation and expansion. However, discovery and development of agonistic antibodies is much more challenging than antagonistic antibodies.
Antagonistic Receptor Blocking
BsAbs can engage in antagonistic binding by occupying cell surface receptors and inhibiting their activation. For example, a bsAb was designed to inhibit immune checkpoint receptors programmed death protein 1 (PD-1) signaling through dual binding of PD-1 and
cytotoxic t-lymphocyte associated 4 (CTL4). This dual antagonistic binding and tethering activity led to the internalization and degradation of PD-1, consequently enhancing T-cell activity and promoting anti-tumor immune responses.
Barrier Translocation
The blood-brain barrier selectively allows certain compounds to pass through, actively blocking the majority of molecules, particularly biotherapeutics and antibodies. However, some bsAbs have been developed to overcome this barrier by utilizing receptor-mediated transcytosis, binding specifically to the transferrin receptor (TfR). An approach to increase antibody exposure to the brain involved designing a DVD-IgG, which targets both the TfR and the therapeutic target—a protofibrillar Aβ peptide. Dual binding to TfR and the Aβ peptide increased brain and plasma exposure compared to a monospecific anti-Aβ antibody.
De Novo Sequencing and Antibody Characterization
Bispecific and multispecific antibodies present significant challenges in antigen targeting, mechanism of action, engineering, production and developability.
- Discover novel antibodies with REpAb® antibody discovery service using mass spectrometry to analyze the entire circulating antibody repertoire.
- Inform your engineering process with the complete amino acid sequences of candidate mAbs with REmAb® de novo antibody sequencing service to construct your bsAbs and msAbs.
- Confirm binding epitopes, epitope binning, and binding kinetics of engineered bsAbs and msAbs with HDX-MS and SPR.
BsAbs and msAbs are notoriously difficult to produce, involving engineered sequences, peptide linkers, and post-translational modifications like additional disulfide bonds. It’s challenging to ensure the intended production.
LC-MS-based antibody characterization can address these uncertainties, providing confidence in high-quality bsAbs and msAbs production with accurate sequences, disulfide linkages, and appropriate post-translational modifications.
Ready to embark on your bispecific development journey? Contact our team of scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

