Antibody Characterization Services.
Our antibody characterization services are built on deep expertise working with antibodies, high throughput mass spectrometry and advanced software. Our services are tuned for speed, to give critical insights in the later stages of discovery and early stages of pre-clinical production.
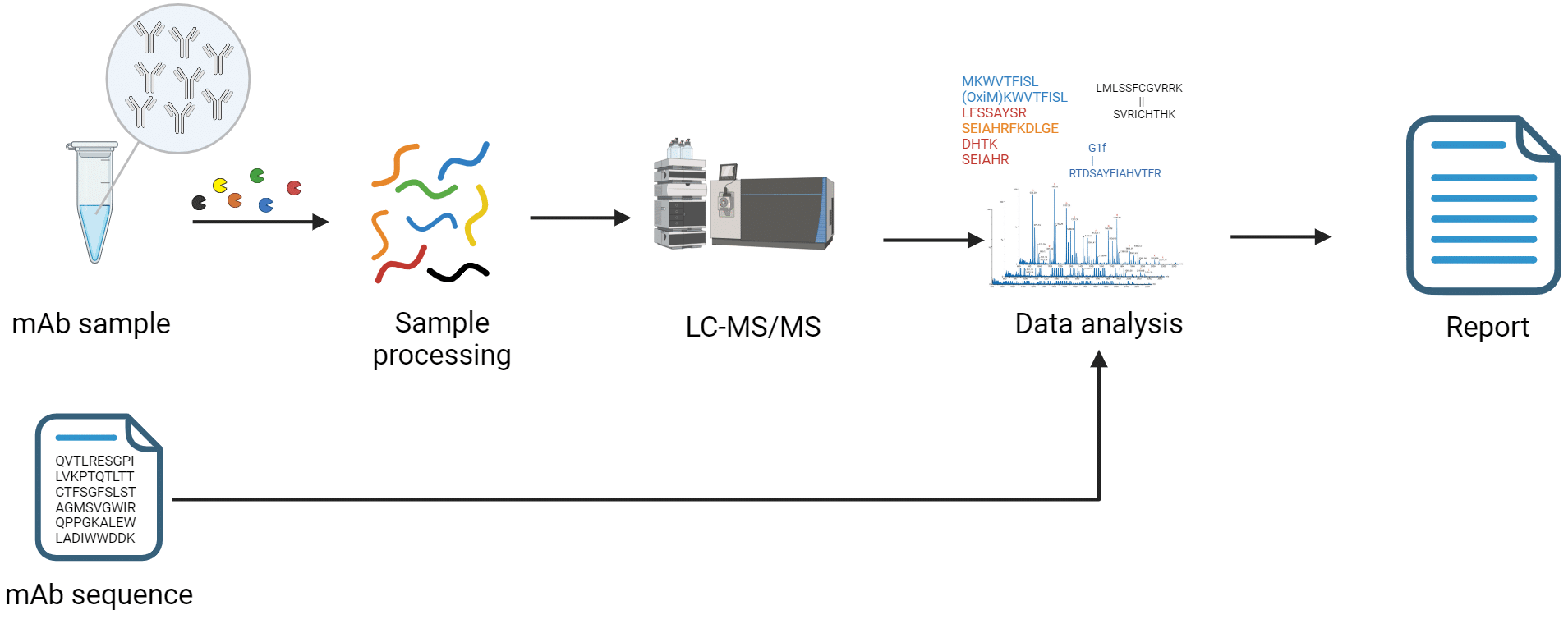
How Antibody Characterization by LC-MS Works.
While there are many ways to characterize an antibody, there are a few that are critical in discovery stages and early production. Rapid Novor specializes in Liquid Chromatography Mass Spectrometry (LC-MS) antibody characterization because of its speed and accuracy. Several types of analysis are possible, with differences in the sample preparation and data analysis, depending on the information needed.
The typical analyses involve trypsin digestion and analysis by LC-MS/MS. Intact or sub-unit analysis is also possible.

Intact Mass Analysis.
Among the most versatile tools for antibody characterization, intact mass analysis by LC-MS can be used for a variety of applications. Researchers can quality control antibody production, examine glycosylation, conjugation or other modifications. Intact mass analysis is also often used as a first step in troubleshooting performance issues. It can be detailed and accurate, or high-throughput and low cost.

Disulfide Bond Analysis.
Disulfide bonds are what hold the different parts of the antibody together. They form between pairs of cysteines. If you engineer a sequence, the resulting antibody may or may not have the disulfide bonds you want – the bridge might not form or might connect the wrong parts together some of the time. Discovery scientists and protein engineers may analyze disulfide bond shuffling in order to select or reject mAbs for downstream work. Production teams who have access to this information early in their process can have higher success rates downstream.
PTM Analysis.
Different production, handling and storage conditions can cause different levels of oxidation, deamidation, truncation and clipping of the protein. These modifications are generally termed post-translational modifications (PTM). Optimizing production, handling and storage requires differential analysis of PTM. Similarly, discovery scientists may select or engineer sequences that are less susceptible to PTM. PTM analysis can determine sequences or production conditions that are more favorable.

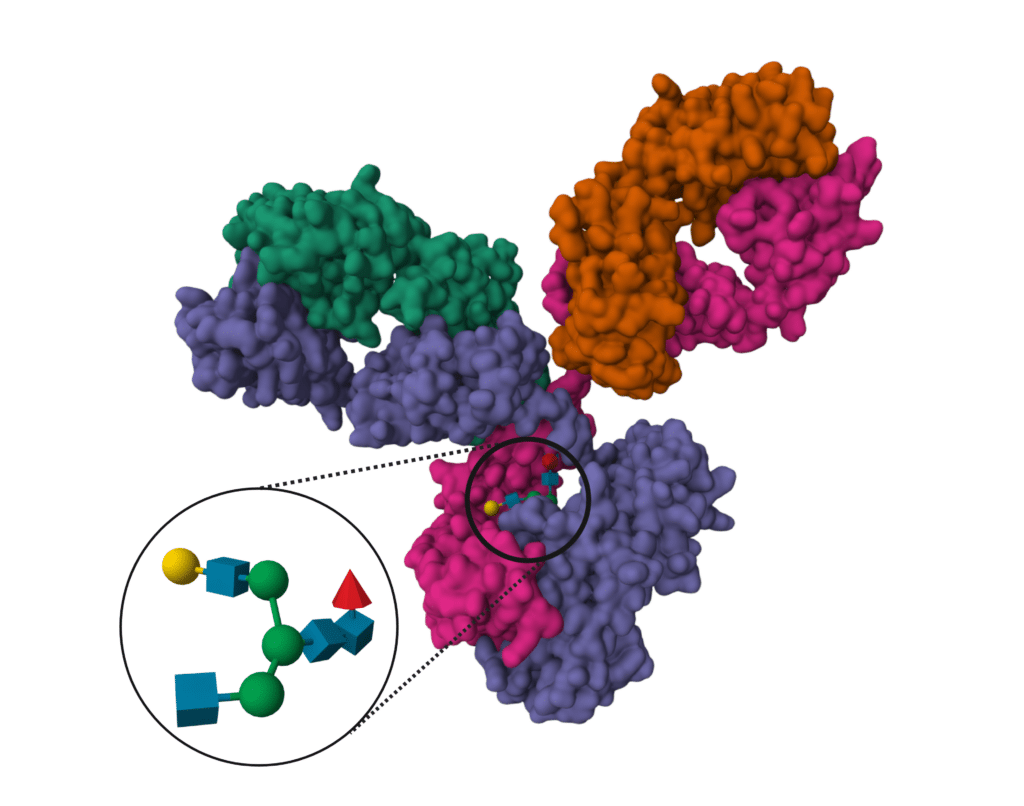
Glycosylation Analysis.
Glycosylation contributes to the stability and function of a mAb. Fab glycans are common but often overlooked, and can be problematic for production, so discovery teams may use glycosylation analysis to fully understand and select lead molecules. Production teams are careful to examine the profile and location of glycans as they consider new cell lines or scales of production.
Sequence Variant Analysis.
Although recombinant expression is reliable, errors in production are still possible and must be identified very early in the process to avoid wasted efforts. Single point mutations can occur, portions of the sequence can be reversed, or deficiencies in the growth media can cause amino acid substitutions. Additional chains can be produced in error. Contaminants can be introduced. Identifying these issues early on can help troubleshoot performance problems and prevent future errors in production.


Deliverables.
Peptide Mapping
- Peptide map
- % coverage achieved
- List of observed peptides
- Interactive coverage viewer and coverage map
- Any other observations for further investigation
Intact Mass Analysis
- Mass spectrum
- Deconvoluted mass spectrum
- Analysis report
Glycosylation Analysis
- Glycan site occupancy report
- Glycan profile (i.e. g0, g0f, g1, g1f, etc. – % values that sum to 100)
Drug-Antibody Ratio Analysis
- Mass spectrum
- Deconvoluted mass spectrum
- DAR value
- Analysis report
Disulfide Bond Analysis
- Sequence annotated with disulfide bonds
- Confidence measure on each
- Prevalence of each (percentage)
- [Comparison of samples]
Sequence Variant Assessment
- List of peptides that closely match the expected sequence
- % of each variant (by peak area)
- List of peptides that match common contaminants
- Rough assessment of quantity of non-matching peptides
PTM Analysis
- Sequence annotated with PTM names
- Prevalence of PTM at each AA position (calculated by peak area)
- Identification of C-terminal or N-terminal truncation
- [Comparison of samples]
- [Optional D-isomerization analysis]
Custom Analysis
- Please inquire about your custom analysis needs
Getting Started.
Requirements
- Amino acid sequence(s) in FASTA format
- 50 μg per analysis per sample
- >90% purity
- Monoclonal antibody (any isotype, format or fragment)
- [List of PTM of interest]
Timeline
Screening
“High quality, reliable work, exceeding expectations.”
– JJ, Protein Science Consultant
(LC-MS Antibody Characterization Project)
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics
Talk to our scientists. We have sequenced over 9000+ antibodies and we are eager to help you.
Antibody Sequencing, Discovery and Characterization Services.
Antibody Sequencing & Discovery Services.
REmAb® mAb Sequencing
Monoclonal antibody sequencing from small antibody samples, no need for hybridoma or DNA information. Full sequence in record time.
Explore Antibody Sequencing Services
REpAb® pAb Sequencing
Polyclonal antibody sequencing and antibody discovery service. Sequence antibodies from blood or a polyclonal mixture.
Explore Antibody Discovery Services
RapidSPR™ Analysis
Label-free evaluation of antibody-antigen binding using surface plasmon resonance on Biacore or Nicoya. Can be employed for detailed kinetic profiling, kinetics screening, or epitope binning.
Explore SPR Services
RapidHDX-MS™
Mass spec based epitope mapping. Epitope mapping for identify the binding site of an antibody to its corresponding antigen with the highest confidence and resolution.
Explore HDX-MS Epitope Mapping Service
MATCHmAb™
Antibody verification by rapid peptide mapping. Antibody sequence confirmation and full detailed mapping for development purposes.
Explore Peptide Mapping Service
Antibody Characterization
LC-MS analysis for study of antibody glycosylation, disulfide bonds, post-translational modifications, sequence variants, intact mass and size.
Explore Antibody Characterization Services