Biosimilar Development.
Comparability Analysis for Biosimilars.
Biosimilars are biologic drugs that closely resemble an approved reference drug with no clinically significant differences. Biologics are intricate molecules produced from living cells, making it difficult to replicate them identically. Instead, biosimilars are designed to be highly similar to the reference product in terms of structure, efficacy, safety, and quality.
Analyzing the protein sequence of the reference biologic allows for a comparative assessment of the biosimilar’s structure and performance, while proteomics techniques offer insights into its physicochemical and biological properties.
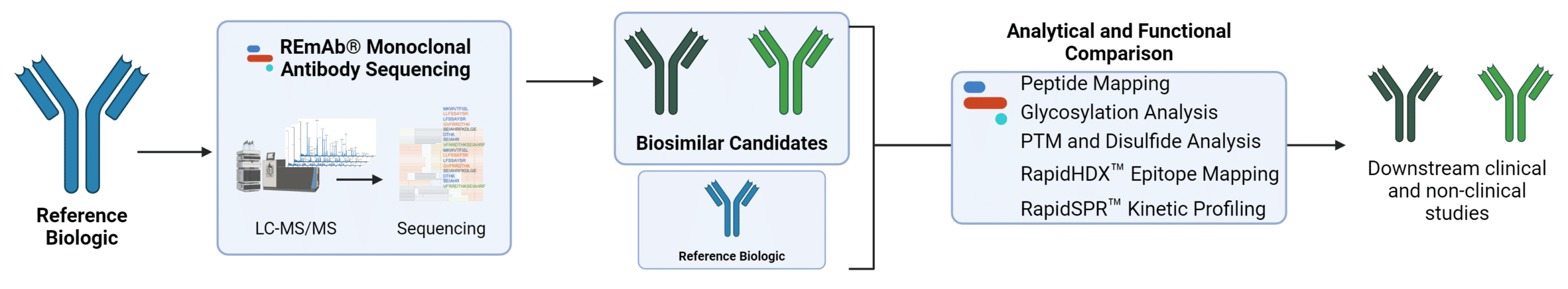
Services for Biosimilar Development.
We support the characterization process for biosimilar product development. Contact us to discover how we can help with your biosimilar development application.
De Novo mAb Sequencing
Obtain the protein sequence for the marketed mAb therapeutic as a foundation for the development and characterization of the biosimilar product.
Explore Antibody Sequencing Service
Comparability Analysis of Sequences
Identify sequence variations and demonstrate similarities/differences between the biosimilar and the reference mAb with peptide mapping.
Explore Peptide Mapping Service
HDX-MS Epitope Mapping
Examine or validate biological targets of the biosimilar and reference mAb by characterizing their antigenic epitopes.
Explore HDX-MS Service
SPR Binding Kinetics
Obtain insight into the physicochemical properties of the biosimilar and reference mAb by characterizing the antibody-antigen binding kinetics.
Explore SPR Analysis Service
Analysis of Post-Translational Modifications
Identify crucial post-translational modifications (PTMs), disulfide bonds, and glycan profiles of biosimilar candidates to facilitate comparative functional evaluations and subsequent development processes.
Explore Antibody Characterization Services
Recombinant Antibody Expression
Compare structure and performance of the expressed biosimilar against the reference mAb in a streamlined workflow combining proteomics and recombinant expression.
Explore Antibody Expression Service
Application Publications.
“We’re focused on coupling cytokines with a highly engineered VHVL of an antibody. So by sequencing the antibodies with Rapid Novor we are able to pull out the CDR to graft them into our platform and test in vitro and in vivo.”
Pavel Khrimian – Co-founder and CBO, Deka Biosciences
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics