 Written by:
Written by:
Genya Gorshtein, MSc
Published: April 21, 2023
Introduction
Intellectual property (IP) protection is a critical step during the commercialization of antibodies for therapeutic and diagnostic purposes. Requirements to obtain IP rights for antibodies may vary between countries or regions but general requirements include:
- Novelty – The antibody must be new and not previously disclosed or made available to the public prior to patent filing.
- Inventive step/Non-obviousness – The antibody must not be obvious to those skilled in the art and must have a technical effect.
- Sufficiency – The patent application must include a written description, which allows replication of the invention.
There are many strategies and considerations for securing patent protection on antibodies, due to their complexities and rapidly changing technologies in the industry. Some claim approaches to protect antibodies may be specified by:
- Target Antigen: e.g. antibody X that binds specifically to antigen Y.
- Structure: claiming the antibody CDRs or more complete sequence.
- Function: e.g. binds to an antigen with a particular binding affinity or induces apoptosis.
- Target Epitope: e.g. antibody X that binds specifically to amino acid sequence…
- Hybridoma accession number: e.g. antibody produced by the hybridoma cell line deposited under the accession number…
- Method of production: e.g. antibody produced by immunization on a non-human with a peptide of SEQ ID NO: X.
As IP offices (i.e. USPTO, EPO) place immense scrutiny on antibody-related patent applications during the prosecution process; preparation prior to patent filing is key to a successful application. It is important to be prepared with essential information and data on the claimed antibodies, as it is often difficult to add additional information once the prosecution begins.
Target Specification for First Generation Antibodies
Specification by target is similar to compound protection claims used for small molecule pharmaceuticals and may provide the broadest scope of protection. Compound protection by target specification is generally used for first generation. However, the most important protein targets in human therapies have been well characterized for decades. As such, claiming antibodies in this way may place a greater burden on applicants to demonstrate that the antibody exhibits an unexpected property, for example. As such, recently developed antibodies are considered second or higher generation antibodies and patent applications may be required to use more restrictive claim language.
Patent Strategies for Second-Generation Antibodies
Second-generation antibody patents are granted to antibodies that are considered novel and inventive, against a target that is already known. The novelty requirement of the claimed antibody is met if the antibody has unique or improved properties and functions. Defining an antibody by its structure and amino acid sequence, and by binding characteristics, such as epitope, affinity, and avidity can be possible options:
Structure and Sequence Specification
Specification by amino acid sequence of the claimed antibody provides a narrow, but clearly defined scope of protection. Amino acid sequence specifications for antibody patents may discourage the emergence of “biosimilars” in the market – as biosimilars are classified by having an identical amino acid sequence.
Sequence specification often includes reference to one or more complementarity determining regions (CDRs) of heavy and light chains of the immunoglobulin. A majority of antibody claims disclose the entire immunoglobulin sequence or 6 CDR sequences in claim. Antibody claims that are defined by sequence and CDR structures may also be coupled with a degree of flexibility for sequence identity and functional limitations. For example, a claim can define antibody sequences with 90% sequence identity to the disclosed sequences of the antibody. Additionally, some sequence claims also restrict the sequence to a specific functional capability of the antibody, such as neutralizing or inhibiting activity.
Target Epitope
Describing the epitope for the claimed antibody can be used to fulfill the requirements of patent law. Additionally, epitope information may allow for freedom-to-operate rights, as binding epitopes on the antigen can differentiate between later-generation and earlier-generation antibodies that target the same antigen. If it is demonstrated that they have distinct binding epitopes, an antibody can target the same antigen as another antibody already patented. However, an existing antibody that binds to the same epitope and produces the same effect or outcome, even if the epitope was not previously described, can undermine the novelty of the claim.
Function by Binding Kinetics
Kinetic analysis can be used to define a claimed antibody with improved functional properties, based on its affinity, avidity, and binding kinetics. Claims such as these require additional evidence to describe the improved property of the antibody, and how it was developed in order to support the inventive step requirements. For example, affinity claims can define an antibody by improved affinity for its target by affinity maturation processes. Improved affinity for the target antigen can thus provide a greater therapeutic advantage. Typically, improved antibody kinetics are the result of alterations to the CDR sequences or antibody structure, which should be communicated as well.
Production Processes and Hybridoma Deposits
In combination with sequence specification, antibodies may be claimed based on a deposit of biological material from which the antibody can be produced from, such as a hybridoma cell line. These claims may refer to only part of the deposited antibody, such as a specific CDR domain. As such, the patent may extend to structural variations such as humanized or chimeric antibody formats derived from the same hybridoma cell line. Methods of production, such as immunization regimes or expression systems may also be used to claim antibodies. These claims are referred to as “product-by-process” which describe the methods and materials used to generate the resulting antibody.
Next Generation Protein Sequencing and Proteomics Strengthens IP Protections
Antibody patents based solely on functional features or amino acid sequences may provide a scope of protection that is easy to design around. Patents that reference a combination of antibody characteristics, such as amino acid sequence, binding epitope, affinity, and avidity can make life harder for competitors. As such, utilizing NGPS and proteomics technology together can provide relevant structural and functional details of antibodies for preparing patent applications (Figure 1).
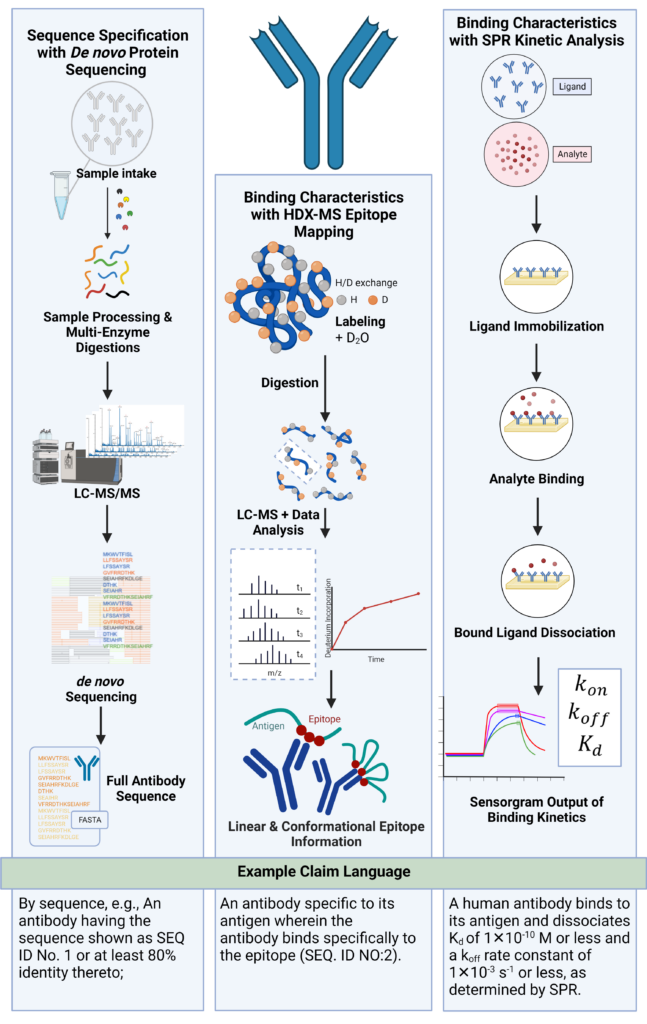
Figure 1. De novo protein sequencing, epitope mapping, and SPR binding kinetics characterizes antibodies and provides essential information for patent protection. On the left, depicts the workflow for REmAb® for de novo monoclonal antibody sequencing. In the middle, depicts the workflow for HDX-MS epitope mapping to obtain linear and conformational epitope information. On the right, depicts the workflow for SPR kinetic analysis to obtain binding kinetic information. Utilizing the generated data, patent claim language can be used to protect the antibody based on its novel characteristics and functions.
Next-Generation Protein Sequencing
De novo protein sequencing uses liquid chromatography-mass spectrometry (LC-MS) to derive amino acid sequences directly from the mass spectral data without prior knowledge of the sequence. Utilizing de novo sequencing for monoclonal antibodies can resolve 100% sequence including all CDRs with 100% accuracy, critical for claiming sequences for antibody patents. For more information about our REmAb® technology, visit our antibody sequencing services.
Epitope Mapping with HDX-MS
The key to describing epitope binding in patent claims is by utilizing high resolution epitope mapping technology, such as Hydrogen-Deuterium-eXchange Mass Spectrometry (HDX-MS) to identify key amino acid residues required for antibody binding. HDX-MS can provide high resolution of 1 to 5 amino acids to accurately determine linear, conformational, and structural binding epitopes. For more information, visit our HDX-MS epitope mapping services.
Kinetic Analysis with SPR
Kinetic analysis by Surface Plasmon Resonance (SPR) can provide unmatched accuracy and reliability on binding affinity, on-rates, and off-rates (KD, Kon, Koff) of antibody antigen interactions to claim improved functional properties. For more information, visit our SPR kinetic binding analysis services.
To learn more about our services, inquire with our scientists.
For more information about navigating IP protections for antibodies, watch our previous webinar co-hosted by: Kirsty Dolphin, PhD, CPA, EPA; Partner and Patent Attorney at Venner Shipley.
Disclaimer: This article is for promotional purposes only, and it is not legal advice. Please consult your regional legal counsel for more information on IP and antibody patent filings.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

