Antibody Engineering.
Informed Engineering with De Novo Antibody Sequencing and Proteomics.
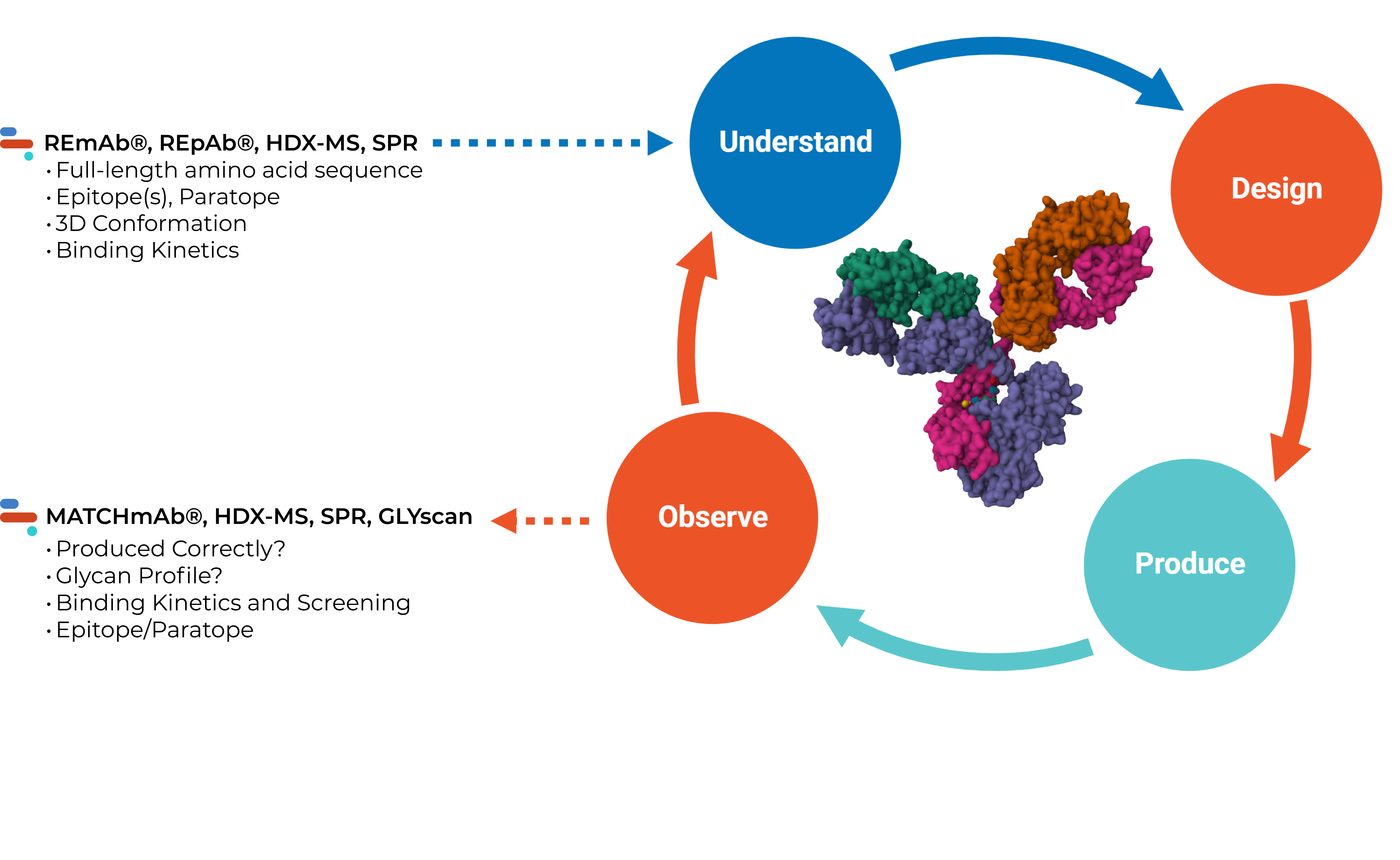
Antibody engineering is rarely a straightforward journey; it can be viewed as a cyclical process of understanding, design, production, and observation. These stages form the foundation of an informed engineering process, accelerating the development of antibodies for diverse applications.
Gaining a deeper understanding of the starting material, including complete antibody sequences, binding kinetics, binding epitopes and 3D conformations offers essential details to fuel the engineering process.
An informed engineering process requires in-depth characterization on the performance of the resulting engineered antibody. Does the antibody bind to the intended epitope? What are the binding kinetics of the antibody? Was there a change in the glycan profile? How well was it produced? Addressing these questions ensures the effectiveness of engineered antibodies, enhancing their utility in therapeutic and diagnostic applications.
De novo antibody sequencing, epitope mapping with HDX-MS, kinetic analysis with SPR, and glycan analysis can answer these questions to better inform your antibody engineering process.
Services for Antibody Engineering.
We support the antibody engineering efforts of life science companies in all stages of development. Contact us to discover how we can help with your antibody engineering application.
Antibody Sequencing & Discovery Services.
De Novo mAb Sequencing
Obtain amino acid sequences of functional antibodies to facilitate in silico design, rational engineering, humanization, chimerization, affinity maturation, conjugation design and construction of fragments and multi-specifics.
Explore Antibody Sequencing Services
Convert a pAb to a mAb
Convert a polyclonal antibody to a monoclonal antibody by obtaining the sequence of antibodies present in a pAb mixture.
Explore Polyclonal Sequencing Services
Peptide Mapping Service to Validate Engineered mAbs
Confirm protein sequences and pinpoint the occurrences of any modifications, mispairings, or mutations after protein expression or during cell line development. Minimize troubleshooting efforts with real-time peptide mapping.
Explore Peptide Mapping Services
SPR Binding Kinetics
Test, assess, and evaluate mAb binding kinetic properties (ie. specificity, affinity, efficiency) to evaluate and reevaluate engineering strategies.
Explore SPR Services
HDX-MS Epitope Mapping
Characterize antigenic epitopes for the rational design of mAbs, scFvs, and multi-specifics. Determine antibody binding sites on target antigens for FDA and EMEA regulatory filings and IP protection.
Explore Epitope Mapping Services
Antibody Characterization Services
Our deep expertise working with antibodies, high throughput mass spectrometry and advanced software will give you critical insights in your antibody.
Explore Antibody Characterization Services
Application Publications.
“We’re focused on coupling cytokines with a highly engineered VHVL of an antibody. So by sequencing the antibodies with Rapid Novor we are able to pull out the CDR to graft them into our platform and test in vitro and in vivo.”
Pavel Khrimian – Co-founder and CBO, Deka Biosciences
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics