 Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: March 5, 2024
Introduction to Flow Cytometry
Flow cytometry is used by a wide variety of industry professionals for applications ranging from biomedical research to clinical diagnostics. Flow cytometry is a very powerful tool that allows users to identify and characterize single cells within a population of cells. Industry professionals exploit this function for many different applications such as measuring the quantity of a protein of interest, assessing the health of a cell population, analyzing specific post translational modifications, and detecting a subpopulation of cells within a complex sample. Flow cytometry can be coupled with fluorescence activated cell sorting (FACS), which can be used to collect specific cells from populations. However, in this blog we will only delve into the intricacies of flow cytometry.
Understanding Flow Cytometry
A flow cytometer is made up of three components that work together to separate, interrogate, and categorize single cells within a sample. These systems are referred to as the fluidics system, optics system, and electronics systems. Here’s how it works (Figure 1):
- To begin, the sample is incubated with fluorescently labeled antibodies that are specific to a cell surface or intracellular marker of interest. This allows researchers to quantify cells based on their unique antigenic profiles.
- Next, cells are resuspended in a sheath buffer where they are transported through the fluidics system to the flow cell where they are separated into single cell droplets.
- As the cells flow through the flow cell, they pass through the optics system which hits the cells with laser beams of different wavelengths.
- When the laser beam passes through the cell it emits forward scatter, side scatter, and fluorescence scatter which are all captured by detectors. Forward scatter (FSC) measures the size of the cell, while side scatter (SSC) measures the fluorescence intensity and internal complexity.
- The detectors convert the scatter into signals that are proportional to the measured amount of light or fluorescence which is delivered to the electronics system for software analysis. The data generated from the software analysis can infer cell type and quantity of cells containing the target(s) of interest.
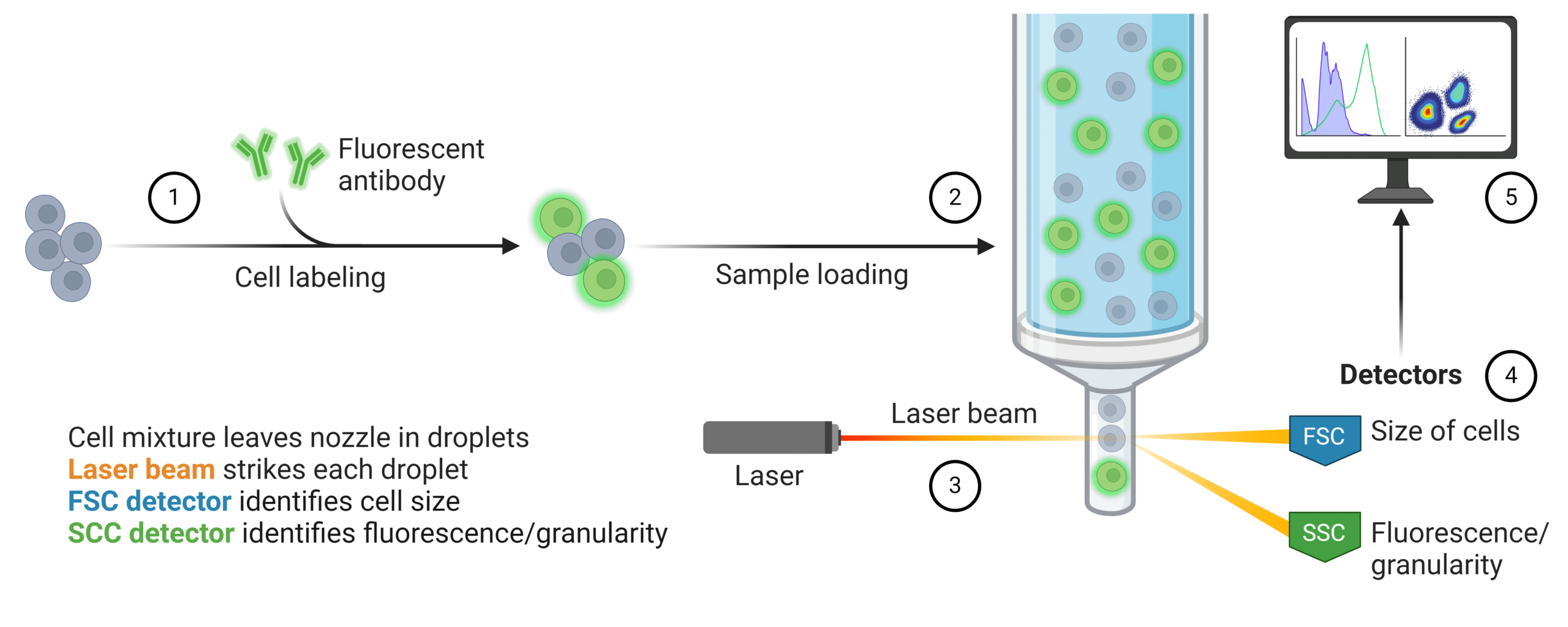
Figure 1: Quantification of fluorescently labeled cell population within a heterogeneous sample using flow cytometry.
Interpreting Flow Cytometry Data
To begin interpreting flow cytometry data, a plot of FSC versus SSC should be created to show the different distinct cell populations in the sample. This allows you to select or “gate” for the population of interest, such as live cells, dead cells, immune cells, or cells expressing your marker of interest. Once you have selected your specific population of interest you can further analyze that subpopulation with your fluorescent marker to identify your cells of interest.
For example, the figure below illustrates what the data would look like after running a whole blood sample on a flow cytometer (Figure 2). As you can see, plotting FSC versus SSC allows separation of debris from the distinct cell populations in blood such as granulocytes, monocytes, and lymphocytes. If a scientist is interested in isolating and analyzing B cells, they would start by selecting or “gating” the lymphocyte population. Since they are only interested in B cells, they would stain the sample with a fluorescent antibody that is specific for a B cell marker such as CD19. To isolate the B cells from the T cells they would also stain for CD3 which is a T cell marker. The scientist could then plot CD3 versus CD19 fluorescence which would separate the T cells and B cells into distinct populations. Gating for the B cell population would then allow the scientist to either count the B cells or measure the B cell expression of a protein of interest.
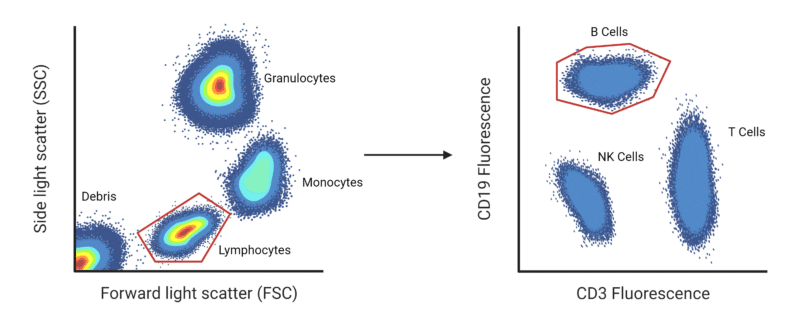
Figure 2: Illustration of B cell separation from a whole blood sample using flow cytometry.
Antibody Selection for Flow Cytometry
In flow cytometry, antibodies serve as indispensable tools for targeting specific antigens expressed on the surface or within the cells of interest. These antibodies are conjugated with fluorescent dyes, allowing for the visualization and quantification of target molecules through fluorescence detection. Researchers must consider a wide range of factors when selecting an antibody for use in flow cytometry to ensure accurate and reliable results.
Factors to consider when choosing an antibody for flow cytometry:
Specificity
It is important to choose an antibody that is specific to the antigen of interest and that minimizes cross-reactivity with closely related proteins. Moreover, carefully choosing the epitope that the antibody binds to is vital since proteins can have multiple different isoforms, splice variants, and post-translational modifications. Epitope mapping can identify the binding site of an antibody to its corresponding antigen. HDX-MS can map complex epitope binding with the highest confidence and resolution.
Clonality
Polyclonal antibodies (pAbs), monoclonal antibodies (mAbs), or recombinant monoclonal antibodies (rAbs) can all be used for flow cytometry. pAbs bind multiple different epitopes and produce very strong signals, but also exhibit cross-reactivity and suffer batch to batch variability. mAbs recognize a single epitope on the target antigen which makes them highly specific and have very low cross-reactivity, but provide a weaker fluorescence signal than a pAb. Recombinant monoclonal antibodies (rAbs) can be used to synthesize a mAb cocktail with antibodies specific to multiple epitopes on the antigen of interest. As a result, rAbs can provide a middle ground between the mAbs and pAbs, offering specificity, reproducibility, low cross-reactivity, and a strong signal.
Multi-Parameter Analysis
One key advantage of flow cytometry is its ability to simultaneously measure multiple parameters on individual cells. By employing panels of fluorochrome-conjugated antibodies targeting different antigens, researchers can perform multi-parameter analyses. Careful design of antibody panels is vital to minimize cross-reactivity and spectral overlap while maximizing signal resolution. Moreover, considering the expression levels and subcellular localization of target antigens is important when designing panels of antibodies for multicolor flow cytometry.
Direct or Indirect Labeling
Depending on the application, scientists can choose to use direct or indirect antibody labeling for flow cytometry. Direct labeling uses a fluorescent primary antibody which requires fewer processing steps and is preferred when doing a multi-parameter analysis since it reduces cross-reactivity. Indirect labeling uses fluorescent secondary antibodies which requires more steps, but can enhance signal intensity, as multiple secondary antibodies can bind to each primary antibody. Moreover, indirect labeling enables the use of primary antibodies raised in the same species, which can be beneficial when designing multicolor flow cytometry panels.
Species Compatibility
Based on the species of your sample, you need to confirm that the antibody used for flow cytometry is compatible. The antibody selected typically originates from a different species than your sample species, especially if you are planning indirect staining. Although the antibodies should be raised in a different species, the primary antibody needs to have cross-reactivity to your sample species to adequately bind your antigen of interest. For indirect staining, the secondary antibody must be raised to bind to the specific isotype of the primary antibody and should not have cross-reactivity to your sample species (Figure 3). As an alternative, chimeric antibodies can be used that have different domains from different species to avoid cross-reactivity and reduce background staining.

Figure 3: Name of secondary antibody that is raised in a mouse to bind rabbit antibodies on the H&L chain of IgG isotypes.
Antibody Validation for Flow Cytometry
Despite advancements in instrumentation and data analysis, standards for fluorescent antibodies remain subpar due to lack of validation prior to use in flow cytometry. Validating antibodies involves rigorous testing to ensure affinity, specificity, sensitivity, and reproducibility in detecting the target antigen. Antibody validation can involve several techniques and methods such as MATCHmAb antibody mapping, surface plasmon resonance (SPR) antibody-antigen interaction assay, knock-out or overexpressing cell lines, immunoprecipitation (IP), immunohistochemistry (IHC), immunofluorescence (IF), western blotting, and enzyme-linked immunosorbent assay (ELISA) (Figure 4). Additionally, it is important to have proper positive and negative controls and isotype controls. As exhaustive tests, you can also treat the antibody with a blocking peptide or omit the primary antibody to ensure specificity and set background fluorescence levels. For more information, check out our antibody validation blog here.
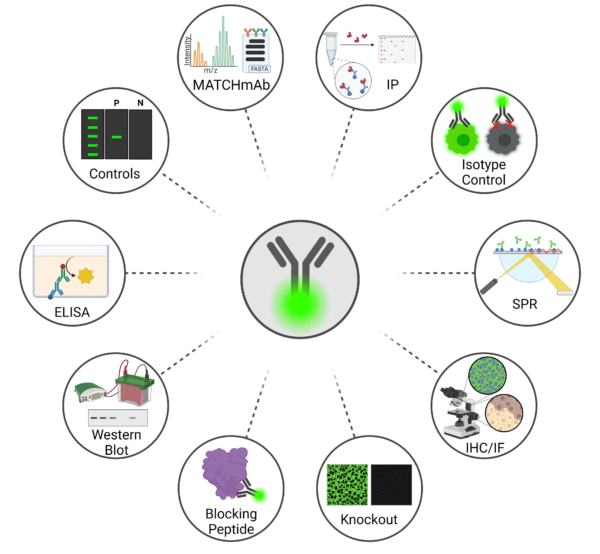
Figure 4: Techniques for Validating Antibodies Prior to Conducting Flow Cytometry.
Antibody Sequencing and Peptide Mapping Services
The importance of going beyond binding assays becomes clear when you consider how important antibody sequence is to the structure and function. In order to perform flow cytometry with the highest caliber of success and reproducibility, obtaining the antibody sequence and sequence conformation with Rapid Novor’s antibody sequencing service and peptide mapping service.
Contact our scientists to learn more about strategies for antibody reproducibility.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

