Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: November 29, 2023
Introduction
Nanobodies, also known as single-domain antibodies or VHHs, are derived from the immune systems of camelids and are heavy-chain-only antibodies. Despite their small size, they possess similar antigen-binding capabilities as conventional antibodies. Due to their unique properties such as tissue penetration, stability, and solubility, nanobodies have played a pivotal role in the development of many therapeutics and diagnostics. There is a vast array of nanobodies that have been developed to target a wide range of molecular targets. However, knowing the precise sequence of nanobodies is crucial to fully harness their potential in developing nanobody-based technologies.
Nanobody Structure
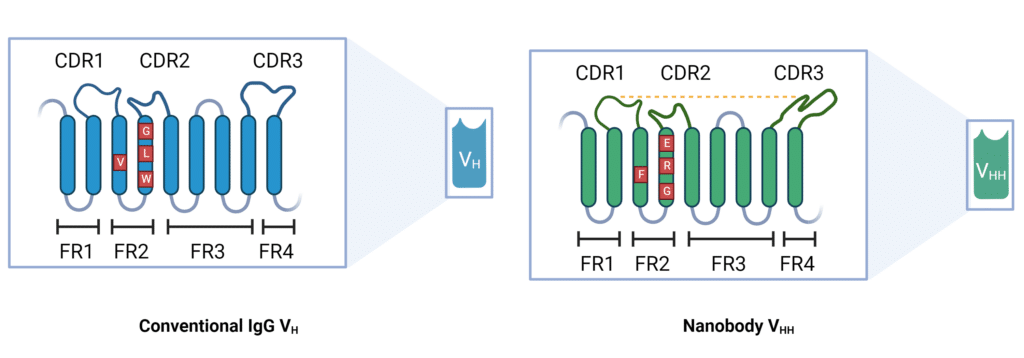
A nanobody consists of a single polypeptide chain that is 110 amino acids long and has a molecular weight of 15kDa (Figure 1). This polypeptide is derived from the heavy chain of an antibody from camelids and contains all the functional domains needed to bind an antigen and elicit a response similar to a traditional antibody. Nanobodies and VH domains of conventional antibodies share many similarities and differences in their structural traits (Figure 2):
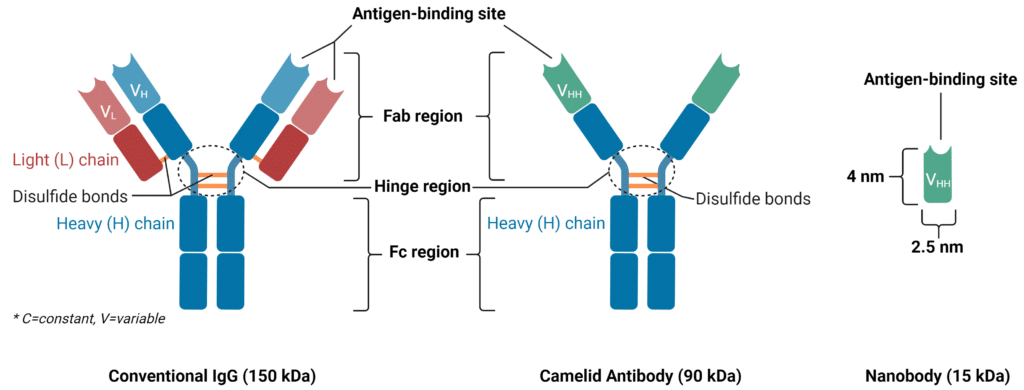
Figure 1: Structural differences between conventional IgG antibody, camelid antibody, and nanobody.
Similarities
- Framework Regions: Consists of four conserved framework regions (FR).
- Complementary determining regions: Have complementary determining regions (CDR1-3) that are highly variable and responsible for antigen binding.
- Folding: Have FR and CDR regions that fold into two β-sheets, one made up of four β-strands and the other of five β-strands. CDRs are located between the β-strands and form the antigen-binding site.
Differences
- Structural complexity: Nanobodies are monomeric entities that only have three CDRs, while the VH domains of conventional antibodies typically have six CDRs.
- Antigen Binding: The reduced complexity in nanobodies allows for an enlarged CDR1 loop and an extended CDR3 loop which both contribute to an increase in nanobody antigen binding diversity (Figure 2).
- Disulfide bonds: VH domains of conventional antibodies contain many disulfide bonds that link them to the VL domains. Nanobodies typically only have a single disulfide bond in the VHH domain that contributes to structural stability.
- Amino acid composition: Due to the necessary interaction of the VH and VL regions of conventional antibodies, the FR2 contains conserved hydrophobic amino acid residues. For nanobodies, the FR2 consists of hydrophilic amino acid residues. This difference is key to understanding how nanobodies exist as insoluble monomers.
- Paratope shape: Due to the extended CDR3 loop in nanobodies, they exhibit a convex paratope shape whereas VH-VL paratopes are concave or flat.
Figure 2: Structural differences between conventional IgG antibody VH domain and Camelid VHH domain.
Nanobody Generation and Production
In recent years, nanobody development and generation have evolved greatly. Below is the workflow to discover, generate, and produce nanobodies using the REpAb de novo sequencing platform (Figure 3).
- Immunization: Camelids, such as camels and llamas, are immunized with a target antigen which triggers an immune response that produces various types of antibodies. Among them, the heavy chain-only antibodies are of particular interest.
- Extraction and Selection: The blood from the immunized camelid is then extracted, and the serum is purified using antigen affinity purification. This method selects for the heavy chain only antibodies that are specific to the antigen of interest.
- Digestion: The isolated heavy chain-only antibodies are digested into peptides using a multi-enzyme digest.
- Sequencing: The peptides are sequenced using liquid chromatography with tandem mass spectrometry (LC-MS/MS). The peptide sequences are used to generate full antibody sequences de novo using machine-learning (ML)-based bioinformatics.
- Recombinant Production: The nanobody sequences are inserted into an expression vector and transfected into a host cell of choice. The nanobody of interest is then produced after expansion, and the host cell undergoes screening to ensure the uptake of the vector. Afterward, it can be scaled up for larger-scale production.
- Purification and Quality Assurance: The produced nanobodies undergo different purification steps, such as affinity chromatography, to remove impurities and isolate the nanobodies of interest. After purification, the nanobody can be put through different quality control measures, such as SDS-PAGE, enzyme-linked immunosorbent assay (ELISA), and sequence confirmation by mass spectrometry.
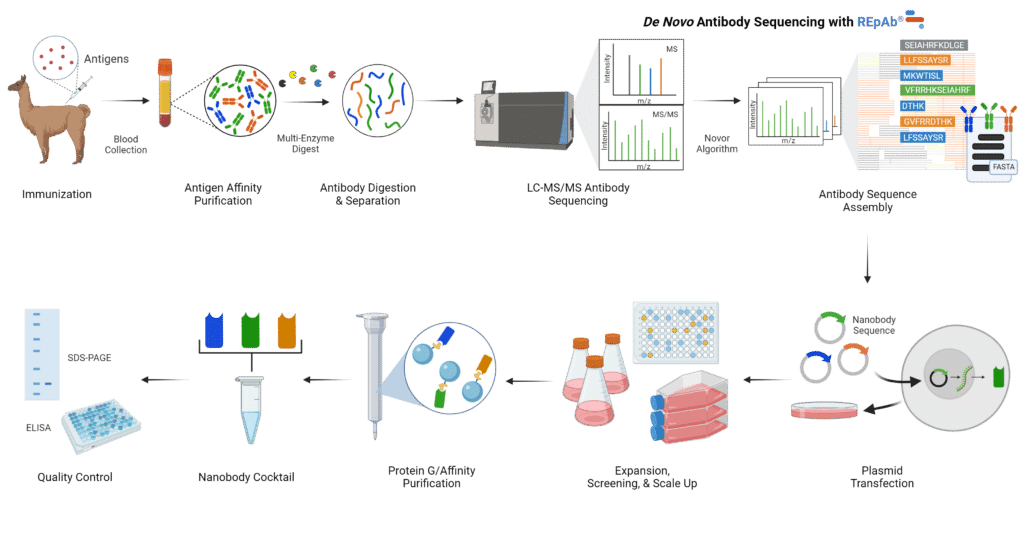
Figure 3: Overview of Nanobody Generation using the REpAb De Novo Sequencing Platform.
Nanobody Applications
The breakthrough discovery of nanobodies by Hamers-Casterman and his team in 1993 has led to remarkable innovation and discovery in the field of biotechnology. Since the discovery of nanobodies, they have become an invaluable tool across many different industries with an emerging wide range of applications in fields such as diagnostics, therapeutics, and research.
Diagnostic Applications
Nanobodies have emerged as incredibly strong diagnostic agents, revolutionizing the way we detect and monitor disease. Immunoassays, such as lateral flow immunoassay (LFIA) and diagnostic ELISAs, have greater diagnostic potential when nanobodies are used instead of conventional antibodies. Nanobodies are ideal for immunoassays due to their increased affinity and stability, greater paratope repertoires, and host antibody cross-reactivity prevention. For example, nanobody immune assays for detecting T. solium have been shown to outperform their conventional antibody counterpart by providing unambiguous test results.
Nanobodies have also been incredibly beneficial to diagnostic imaging methods such as positron emission tomography (PET) and single photon emission computed tomography (SPECT). Nanobodies increase the diagnostic potential of these imaging methods since they penetrate tissue much deeper than conventional antibodies and also have faster renal clearance. Recent studies have shown that nanobodies offer higher contrast PET images as well as less radiation to off-target organs compared to conventional antibodies further highlighting the benefit of nanobodies in the field of science and medicine.
Therapeutic Applications
Nanobodies have unique characteristics that make them excellent candidates for therapeutics. These characteristics include high specificity, affinity, solubility, thermostability, and tissue and cell penetration. Additionally, when compared to conventional antibody therapeutics, nanobodies also have low aggregation, immunogenicity, and serum half-life. They can be engineered to target almost any disease-causing molecular target and can act as inhibitors or neutralizers.
Cancer Therapeutics: Nanobodies are especially advantageous for treating cancer as they can more easily penetrate tumors and can be engineered to target cancer-specific targets. In recent years, there have been many clinical trials involving nanobody cancer therapeutics with three of them already approved for clinical use. For example, in 2022 nanobody expressing CAR T-cells were approved by the FDA for treatment of multiple myeloma.
Autoimmune Therapeutics: Autoimmune disease therapeutics is where nanobodies have had their biggest impact. Since 2018, there have been two nanobody therapeutics approved to treat various autoimmune diseases, and many at the clinical trial stage. For example, in 2019 a nanobody therapeutic was approved by the FDA for treatment of a rare blood clotting disorder. Additionally, nanobodies against TNF-alpha receptors have been shown to increase the safety and efficacy of treatment for rheumatoid arthritis and inflammatory bowel disease (IBD) by delivering therapeutic agents specifically to inflamed tissue.
Infectious Disease: Currently, there are only a few nanobody treatments for infectious diseases that have been approved by the FDA. One FDA-approved nanobody therapeutic is used for respiratory syncytial virus (RSV), while others are still in clinical trial phases. However, researchers have found that nanobodies have better neutralization properties compared to conventional antibody therapies. Nanobodies can target specific inaccessible epitopes that conventional antibodies cannot reach, which makes them more effective. For instance, nanobodies developed against SARS-CoV-2 have shown promise in targeting specific inaccessible epitopes. Furthermore, nanobodies are less susceptible to “vaccine escape” and “mutant escape”, which makes them more efficient in neutralizing the COVID-19 pandemic. Though more research is necessary to bring nanobody treatments to the market, their potential is highly promising.
Nanobody De Novo Sequencing
Nanobodies have revolutionized the field of biotechnology and opened up new possibilities for medical diagnostics and therapeutics. However, to fully unlock their potential, it is necessary to know the exact amino acid sequences to develop and optimize nanobody applications.
One of the leading technologies for nanobody discovery is Rapid Novor’s antibody discovery service, REpAb®. This antibody discovery platform employs tandem mass spectrometry and an advanced proprietary algorithm to sequence antibodies directly from serum or purified protein samples without a database.
Our services expedite nanobody drug discovery by making lead selection more streamlined and improving reproducibility, ultimately bringing nanobody therapeutics to market. For more information on our REpAb services, please contact our scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics