Post Translational Modification Analysis Service.
Identify the position and relative abundance of post-translational modifications (PTMs) using LC-MS. This service is optimized for monoclonal antibodies (mAbs) and related forms.
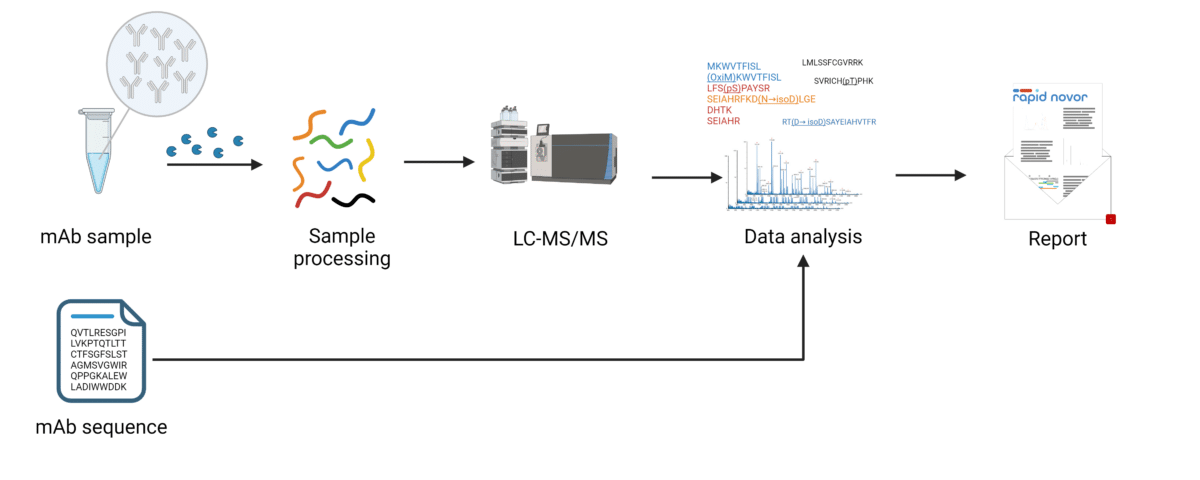
How PTM Analysis by LC-MS Works.
The most widely accepted method for analysis of antibody post-translational modifications uses high pressure liquid chromatography, coupled with mass spectrometry. The primary structure is best evaluated by first digesting the protein into peptides prior to analysis – ‘bottom up’ mass spectrometry. The masses of peptides and fragments of peptides can be matched to theoretical masses from the expected sequence in a process called peptide mapping. Since some amino acid residues are modified after translation, their masses will be affected predictably – for instance deamidation of Asn and Gln, oxidation of Met and Trp, succinimide (N), pyroglutamate. Accounting for these mass shifts allows us to map the modified peptide and its fragments to the expected sequence, and thereby identify the type and location of PTM.
Since both the modified and unmodified forms of the peptide will be detected by mass spectrometry, it is also possible to compare the quantity of modified vs unmodified peptides by looking at their peak areas on the MS1 spectrum.
Why PTM Analysis Matters.
Certain antibody sequences are more prone to post-translational modifications (PTM) than others. Antibody discovery scientists and antibody engineers may select lead antibodies that are less prone to PTM in order to avoid downstream challenges – early and fast characterization by LC-MS helps this process.
Antibody production scientists must determine which production, handling and storage conditions are more likely to cause PTM. In this case, we help our clients to compare between samples produced and handled under various conditions to see which ones have relatively more or less of these PTM.


Which PTMs to look for.
Aside from glycosylation, the most common PTM that affect antibodies are:
- N terminal cyclization
- C terminal lysine truncation
- Deamidation (Asn&Gln)
- Oxidation (Met & Trp)
- Succinimide (N)
Unless otherwise specified, these are included in our analysis. You may select from a list of common PTM, or specify a custom PTM (the amino acids affected and the mass shift must be specified). Glycosylation analysis is a separate service, which can be conducted in parallel.
Focused PTM analysis.
You may choose to scan the whole antibody sequence for all modifications, and quantify each of them. You may choose to analyze the whole antibody for one specific type of modification and quantify its presence at various locations. Or you may choose to focus your analysis on one specific location within the protein. More focused analyses can be completed more efficiently.

Getting Started.
Requirements
- Amino acid sequence(s) in FASTA format
- 50 μg per analysis per sample
- >90% purity
- Monoclonal antibody (any isotype, format or fragment)
- Optional: List of PTM to include in analysis
- Optional: specific location to analyze
Add Ons:
- D-isomerization analysis
Deliverables
- Sequence annotated with PTM names
- Prevalence of PTM at each AA position (calculated by peak area)
- Identification of c-terminal or n-terminal truncation
- Optional: comparison of samples
- Add-on: D-isomerization analysis
Timeline
Screening
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics
Talk to our scientists. We have sequenced over 9000+ antibodies and we are eager to help you.
Other Antibody Characterization Services.
Peptide Mapping
Match the masses of individual peptides to the expected masses from a given sequence, maximizing sequence coverage and confidence.
Explore our LC-MS Peptide Mapping Service
Intact Mass Analysis
Determine the mass of the whole antibody or its sub-units to explore a variety of characterization applications.
Explore our Antibody Intact Mass Analysis Service
Glycosylation Analysis
Find glycosylation site occupancy, and profile their relative abundance %. Compare many samples.
Explore our Glycosylation Analysis LC-MS Service
Rapid PTM Analysis
Locate, identify and quantify post-translational modifications. Compare between samples.
Explore our PTM Analysis LC-MS Service
Sequence Variant Assessment
Identify and quantify variants of your intended antibody within your sample, and consider other contaminants.
Explore our SVA Analysis LC-MS Service
Disulfide Bond Analyses
Analyse peptides cross linked by disulfide bonds. Quantify disulfide bond shuffling in engineered constructs.
Explore our Disulfide Bond Analysis LC-MS Service
DAR Analysis
Determine the ratio of antibody to payload in ADC development.
Explore our Drug-Antibody Ratio Analysis Service
Antibody Sequencing, Discovery and Characterization Services.
Antibody Sequencing & Discovery Services.
REmAb® mAb Sequencing
Monoclonal antibody sequencing from small antibody samples, no need for hybridoma or DNA information. Full sequence in record time.
Explore Antibody Sequencing Services
REpAb® pAb Sequencing
Polyclonal antibody sequencing and antibody discovery service. Sequence antibodies from blood or a polyclonal mixture.
Explore Antibody Discovery Services
RapidSPR™ Analysis
Label-free evaluation of antibody-antigen binding using surface plasmon resonance on Biacore or Nicoya. Can be employed for detailed kinetic profiling, kinetics screening, or epitope binning.
Explore SPR Services
RapidHDX-MS™
Mass spec based epitope mapping. Epitope mapping for identify the binding site of an antibody to its corresponding antigen with the highest confidence and resolution.
Explore HDX-MS Epitope Mapping Service
MATCHmAb™
Antibody verification by rapid peptide mapping. Antibody sequence confirmation and full detailed mapping for development purposes.
Explore Peptide Mapping Service
Antibody Characterization
LC-MS analysis for study of antibody glycosylation, disulfide bonds, post-translational modifications, sequence variants, intact mass and size.
Explore Antibody Characterization Services