 Written by:
Written by:
Vanessa Yoon Calvelo, PhD
Published: June 13, 2022
What is CAR-T Cell Therapy?
The infusion of T cells directed against target antigens as a form of immunotherapy has demonstrated promising clinical responses in patients with hematologic malignancies. One approach garnering attention is the adoptive transfer of autologous peripheral blood T cells that have been genetically engineered to express chimeric antigen receptor (CAR) genes, resulting in the production of CAR-T cells. The overall process of CAR-T cell therapy is described in Figure 1. Briefly, blood is collected from the patient from which T cells are isolated and genetically engineered to express CARs. CAR-T cells are then cultivated and expanded in vitro, followed by CAR-T cell infusion into the patient’s bloodstream for immunotherapy. Along with immune checkpoint blockade therapy, CAR-T cell therapy is shifting the field of cell and gene therapies, providing a promising outlook for patients with refractory cancers.
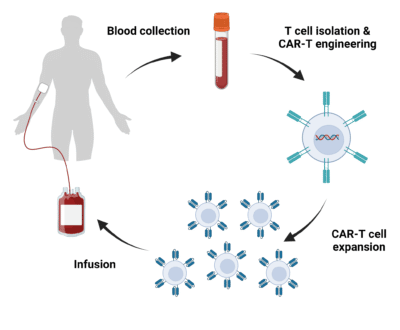
Figure 1. Overview of the CAR-T cell therapy process. Blood is withdrawn from the patient from which T cells are isolated and engineered to express CARs. CAR-T cells are expanded in vitro followed by infusion back into the patient.
CAR Structure and Function
CARs are synthetic receptors that are recombinantly expressed by lymphocytes to recognize and eliminate cells expressing a cognate target antigen. Structurally, they consist of four modular components – an extracellular antigen-binding domain, an extracellular hinge region (or spacer), a transmembrane domain, and one or more intracellular signaling domain(s) (Figure 2).
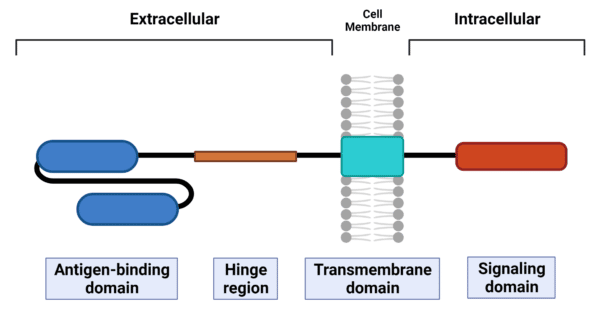
Figure 2. Layout of the CAR structure. CARs have four modular components – antigen-binding domain, hinge region, transmembrane domain, and signaling domain.
The antigen-binding domain usually consists of a single-chain variable fragment (scFv) that is derived from the variable heavy (VH) and variable light (VL) chains of a monoclonal antibody connected by a flexible peptide linker. The scFv connects to the transmembrane domain via the hinge, while the transmembrane domain connects to the signaling domain(s). The transmembrane domain anchors the CAR to the cell membrane and is vital for the stability and expression of the CAR. Upon antigen recognition and binding through the scFv, the signaling domain(s) undergoes conformational changes triggering downstream signaling pathways to elicit an immune response.
CAR-T Cell Development
Based on modifications of the signaling domain, there are five generations of CARs used in the engineering of T cells (Figure 3). First-generation CARs consist of scFvs fused to the TCR signaling component CD3ζ via the transmembrane domain; however, anti-tumour activity of these first-generation CARs is limited due to the lack of costimulatory and cytokine signals. Consequently, second- and third-generation CARs have the addition of one and two costimulatory domains, respectively, to enhance CAR-T cell function. The choice of costimulatory domain(s) affects the efficacy, response phenotype, and metabolic properties of these CAR-T cells. Fourth-generation CAR-T cells, also known as TRUCKs (T cells redirected for universal cytokine-mediated killing), are based on the design of second-generation CARs having one costimulatory domain, but with the incorporation of a separate cytokine signal, such as IL-12. Lastly, fifth-generation CARs, also designed off of second-generation CARs, includes a costimulatory domain with the addition of a cytokine receptor domain, such as IL-2Rβ.
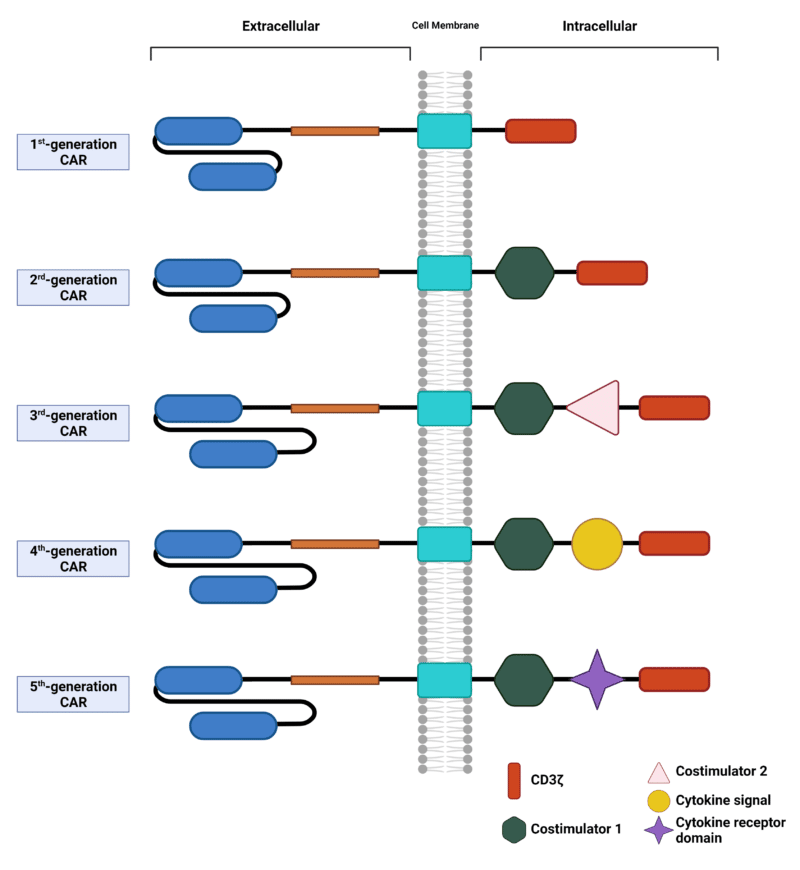
Figure 3. Five generations of CARs. Each generation of CARs are based on modifications of the signaling domain.
Engineering Strategies for CAR-T Cells
As briefly discussed above, five generations of CAR-T cells have been classified based on modifications to the intracellular signaling domain(s). Due to its modular design, however, the other elements within the CAR can be optimally designed to engineer safer and more effective CAR-T cells.
Hinge Region and Transmembrane Domain
Engineering of the hinge region and transmembrane domain influences the stability and function of the CAR-T cells. The hinge provides flexibility to the antigen-binding domain to overcome steric hindrances and facilitate access to the target antigen; therefore, an optimal hinge length is required for effective antigen binding and signaling. Previously, amino acid sequences from CD8, CD28, IgG1 or IgG4 have all been used to derive different hinge regions.
Different transmembrane domains, which are typically derived from type 1 proteins such as CD3ζ, CD28, CD4 or CD8α, have been exploited in the engineering of CARs. As the cell membrane anchor, the type of transmembrane domain used influences the stability of the CAR, and its expression on the T cell. For example, CARs with CD28 transmembrane domains are more stable than those with CD3ζ transmembrane domains.
Antigen-Binding Domain
Traditionally, antigen-binding domains of CARs consist of scFvs that target extracellular antigens of cell-surface proteins expressed by cancer cells. This enables the activation of T cells independent of the major histocompatibility complex (MHC). Various efforts have demonstrated the versatility of engineering scFvs or the antigen-binding domain, as discussed below.
CARs mimicking MHC-dependent T cell receptors (TCRs) that recognize intracellular tumour-associated antigens have been engineered by incorporating scFvs derived from TCR-like fragment antigen-binding (Fab) regions of antibodies. CARs have also been engineered with scFvs that bind to soluble ligands found within the tumour microenvironment. Finally, nanobodies containing the VH domains of camelid antibodies, which inherently lack light chains and thus VL domains, have also been used to engineer CARs.
Modifications to the scFv can influence CAR function beyond antigen recognition and binding. The relative positioning of the complementarity determining region in the scFv can affect the specificity and affinity of the CAR. This ultimately determines the effectiveness of the CAR in T cell signaling and activation, and if not controlled can lead to cell death and other toxicities.
Beyond scFvs, alternative molecules have been exploited as antigen-binding domains for CARs. These include cytokines, such as IL-13 zetakine, ligands, such as a proliferation-inducing ligand (APRIL), and peptides, such as designed ankyrin repeat proteins (DARPins). CARs containing these scFv-alternatives have been tested, or are being tested, in preclinical and clinical studies across a range of malignancies.
De Novo Protein Sequencing Applications in CAR-T Cell Development
De novo protein sequencing is a well-established mass spectrometry-based technique that permits the sequencing of proteins directly from mass spectral data. One advantage to this approach is that there is no requirement for prior knowledge of the peptide amino acid sequences, and no reliance on protein sequence databases.
As an expert in the field of protein sequencing, Rapid Novor’s de novo protein sequencing services can support the design, validation, and manufacturing processes of the CAR-T cell development pipeline. Applications can include:
- REmAb® antibody sequencing services for use in strategic engineering of cell receptors; design and development of enrichment, detection, and validation tools; as well as assays for manufacturing quality control.
- REpAb® antibody discovery services for novel antigen-binding scFvs by examining the functional native immune response and sequencing patient antibodies.
- HDX-MS epitope mapping services for the identification of linear, conformational, and structural epitopes and further binding site characterization.
- Protein engineering and expression of optimal antibodies and antibody fragments for CAR design.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

