Antibody Sequencing Service.
REmAb® has been the world leading antibody sequencing service since 2015, with unmatched throughput and accuracy. Protein de novo Antibody Sequencing determines the amino acid sequence of monoclonal antibodies using mass spectrometry. Our proprietary antibody protein sequencing technology offers full coverage, speed and patented leucine-isoleucine determination techniques.
How Antibody Sequencing Works.
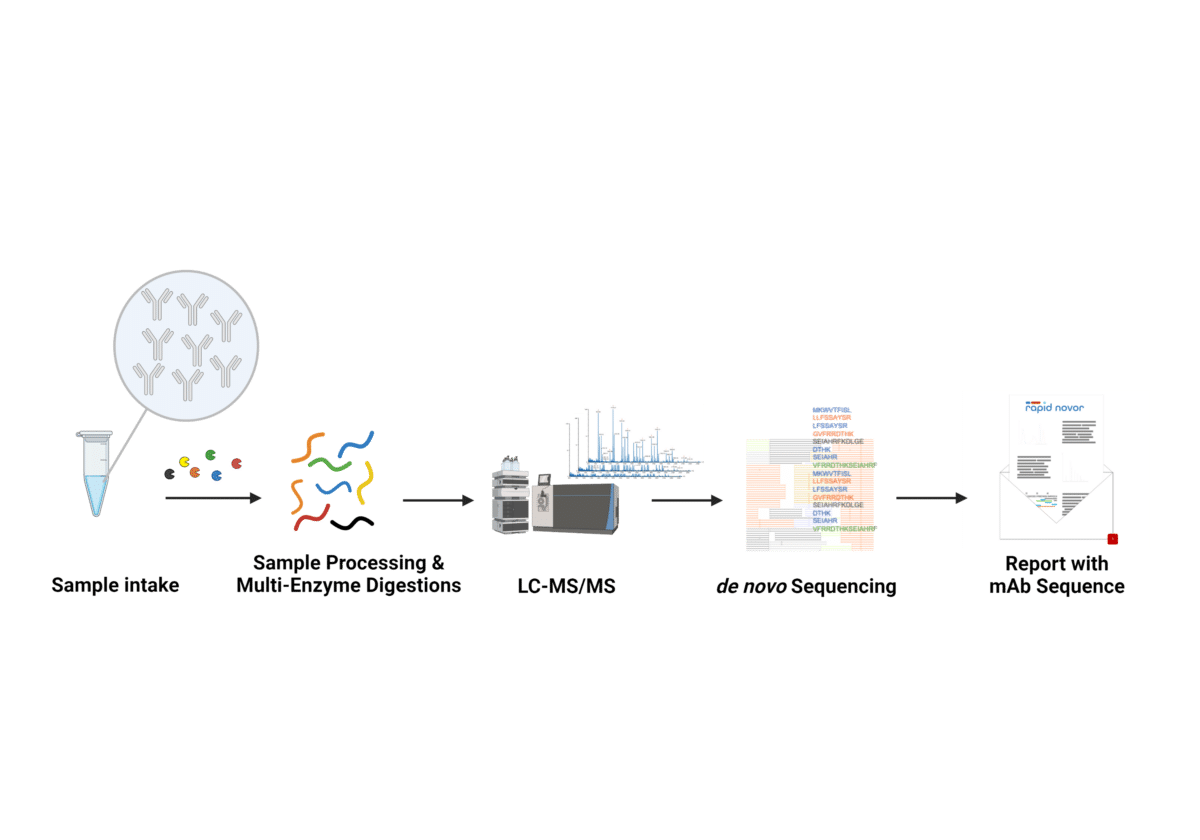
The REmAb® de novo monoclonal antibody sequencing workflow delivers the complete antibody sequence, with unparalleled accuracy and reliability. Starting with just 50μg of purified antibody protein, the sample undergoes multi-enzyme digestions. The fragments are measured with LC-MS/MS for de novo sequencing of the peptides. These peptides are analyzed by Rapid Novor’s suite of bioinformatics algorithms to assemble the full-length heavy and light chain antibody sequences including complementarity determining regions (CDR). Monoclonal antibody sequencing works on any species and isotype.
Our antibody sequencing platform delivers high quality coverage across the full antibody sequence, variable region and constant region, with hundreds of overlapped peptides. This is a de novo sequencing method – it does not require database or sequence data for reference or homology.
| REmAb |
|---|
| Highest throughput: 50 to 100 mAbs per week |
| Highest accuracy: 100% |
| Lowest sample requirement: 50 μg |
| Most enzymes: 5-10 digests |
| Proprietary, next generation algorithms |
| Ile/Leu calls with WILD and WILDtag technology |
| Ability to handle the unexpected |
| Highest coverage: 100X |
| One sequence that works, every time |
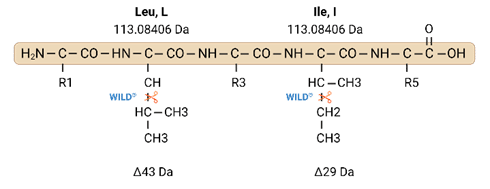
Isoleucine-Leucine Determination.
Our unique and patented WILDtag chemistry increases coverage and confidence in isobaric assignments. Our patented WILD® method was the first commercial service to use additional peptide fragmentation via electron transfer high collision energy dissociation (EThcD) to create and analyze w-ions to resolve isobaric combinations. This enables true Isoleucine-Leucine determination based on experimental evidence alone.
Protein De Novo Sequencing of Any Isotype From Any Species.
We’ve worked with all isotypes and popular constructs, including bispecific, chimeric antibodies, nanobodies and fAbs. Secure 100% coverage of variable domains and obtain antibody sequences that recognize the right antigen with our de novo antibody sequencing service.
World Leading Antibody Sequencing software.
Our team comprises of experienced leaders in mass spectrometry proteomics, protein chemistry and bioinformatics, all under one roof. Our proprietary Novor algorithms provide faster and more accurate sequence analysis, making REmAb® de novo antibody sequencing an important tool for scientists worldwide.
Antibody Sequencing Success Stories.

“You are certainly the best CRO we’ve ever worked with!❤️”

“We’ve focused on coupling cytokines with highly engineered VHVL of an antibody. So by sequencing the antibodies with Rapid Novor we are able to pull out the CDR to grant them into our platform and test in vitro and in vivo”

“Rapid Novor was tasked with finding highly specific, high affinity antibodies against an important target – and they delivered powerful antibodies fit-for-purpose. Such antibodies are not easily obtained yet Rapid Novor approach proved immediately successful, despite our time-constrained and stringent demands”
100% of survey respondents were happy with the experience
Survey respondents were 9.7/10 likely to refer their peers.
85% of survey respondents were already successful downstream. Remainder were in progress. No reported failure.
The REmAb® Advantage.
| REmAb® | Others | |
|---|---|---|
| Throughput | 50 to 100 mAbs per week | Single runs |
| Accuracy | 100% | Often low / ambiguous |
| Sample volume requirement | 50 μg of antibody protein (as low as 7μg) | >150 μg |
| Multi-enzyme digest | At least 5 – 10 enzymes | 1 or 2 |
| Bioinformatics software | Proprietary, next generation algorithms | Previous generation commercial software |
| Accurate Ile/Leu Determination | Yes, WILD® and WILDtag technology | Rarely |
| Ability to handle the unexpected | Yes: additional chains, impurities, oligos, etc. | Rarely |
| Depth of coverage | 100X | Low or patchy |
| Downstream impact | One sequence that works, every time | Costly ambiguities to express and test |
Getting Started with Antibody Sequencing.
Requirements.
- >50 μg of monoclonal antibody
- >80% purity
- Any isotype from any species
Is your antibody sample smaller or less pure? We’ve sequenced many mAbs when the sample amount was much less than our requirements.
Talk to a scientist about your sample.
Deliverables.
- Complete antibody sequence in FASTA
- Interactive peptide coverage viewer and report
- Notable observations
Turn-Around Time.
Antibody Sequencing Packages.
REmAb Gold
- The highest quality antibody protein sequencing available.
- Works with any format, isotype, species or modification.
REmAb Lite
- Ask about our low price, high-throughput service option.
- Designed for pure, monoclonal IgG.
FIRST TIME CUSTOMER? Get a deep discount plus a free upgrade to Gold on all mAbs in your first order.
Optional Add Ons.
Easy Purchasing Options.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics