Background
The native immune system produces polyclonal antibodies (pAb) by different B-cell clones to defend against infectious agents. Compared to monoclonal antibodies, polyclonal antibodies display multiple benefits, including higher sensitivity, higher tolerance, and greater stability. These properties of polyclonal antibodies make them invaluable sources for drug discovery against various diseases and indispensable tools for developing robust immunoreagents for diagnostics.
Yet, the feasibility of accessing and using biologically important polyclonal antibodies remains difficult due to the batch-to-batch variability during production of polyclonal antibodies, and challenges with their biophysical characterization. The most straightforward solution would be to determine sequences of the dominating antibody forms in a polyclonal mixture to enable recombinant antibody generation and ensure reproducibility. This was recently made possible by the development of polyclonal antibody sequencing technology, which will be reviewed in this article.
What is Polyclonal Antibody Sequencing?
Pioneered by Rapid Novor, polyclonal antibody sequencing is an emerging technology that directly derives antibody sequences from blood or a polyclonal mixture. The REpAb® polyclonal antibody sequencing platform was built upon Rapid Novor’s well-established monoclonal sequencing solution (REmAb®) that seamlessly integrates the latest advancements in mass spectrometry-based proteomics and big data bioinformatics.
Polyclonal Antibody Sequencing Workflow
The REpAb® polyclonal antibody sequencing platform seeks to profile, sequence, and characterize polyclonal populations directly from the serum of immunized animals, or a purified polyclonal protein mixture. The platform features a proteomics-based de novo sequencing solution that can derive full-length monoclonal antibody sequences from a complex polyclonal mixture (Figure 1, top panel). The workflow is as follows:
- The serum of an immunized animal with the target antigen or convalescent plasma of a patient is collected.
- The antigen-specific polyclonal mixture from the serum is extracted and purified using affinity purification with chromatography methods.
- The purified polyclonal sample is digested into shorter peptides with multiple proteases.
- The digested peptides are analyzed with liquid chromatography-tandem mass spectrometry (LC-MS/MS) and the sequence of each peptide is determined.
- Using the proprietary AI-based bioinformatic algorithms, the peptide pieces are assembled into longer peptides that belong to individual antibodies.
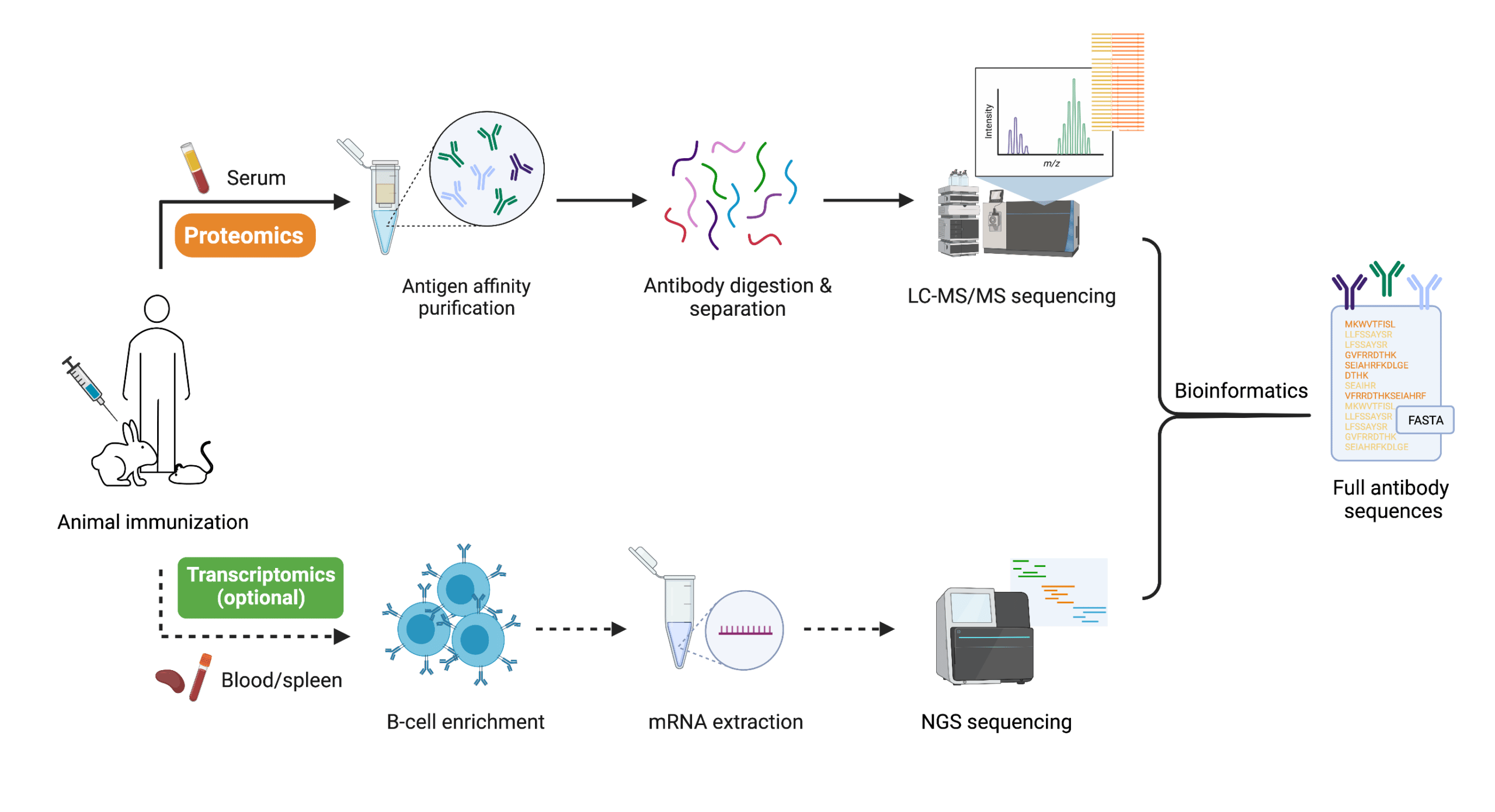
Figure 1. Workflow of REpAb® polyclonal antibody sequencing technology
In parallel to the proteomics analysis, antibody genes in B-cells from blood or spleen can be sequenced with next generation sequencing (NGS) to output transcriptomics data (Figure 1, bottom panel). Once both the proteomics and transcriptomics data are obtained, the two can be combined and cross-referenced to further assemble and derive the complete amino acid sequences of antibodies found in the polyclonal mixture. The sequences can then be used for in vitro recombinant expression to yield antibodies with higher reproducibility for an indefinite amount of time.
Applications of Polyclonal Antibody Sequencing
Polyclonal antibody sequencing technology fits into the drug/diagnostic R&D pipeline at the lead identification and preclinical development stages, such as discovering therapeutic antibodies and diagnostic biomarkers, characterizing post-vaccine immune responses, and developing anti-drug antibodies. Mainly, polyclonal antibody sequencing can be used in the following applications:
- Therapeutic Antibody Discovery – starting with highly expressed functional antibodies, even against hard targets
- Assay development – a fast and direct method for developing antibody-based assays
- IVD development – developing a robust and diverse recombinant polyclonal mixture for increased efficacy and eliminating batch-to-batch variability or supply chain risk
- Vaccine development – characterizing post-vaccine immune responses
- Clinical trials – developing anti-drug antibody assays, biomarker studies
Advantages of Polyclonal Antibody Sequencing
The traditional route for the discovery of therapeutic antibodies involves NGS-based B-cell sequencing, display technology, or hybridoma generation (Figure 2). These approaches have been broadly successful, and recent advances in single B-cell sequencing are promising. However, these approaches aren’t designed to screen for functional antibodies, are reliant on animal models that may not be ideal, or may be liable to immunogenicity challenges.
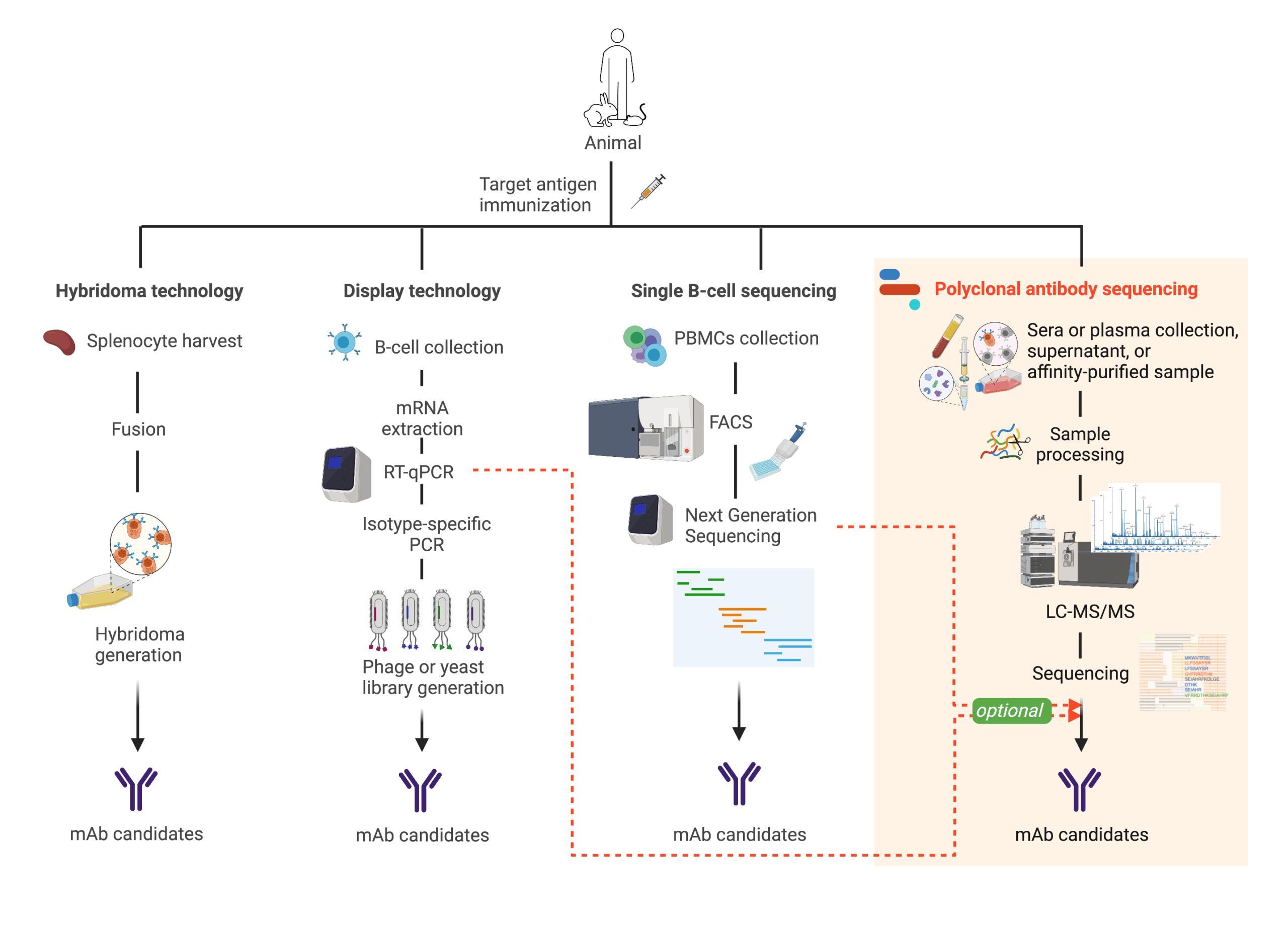
Figure 2. Illustration comparing different antibody discovery technologies
In comparison, proteomics-based polyclonal antibody sequencing reports on the end-point product — naturally occurring and biologically relevant circulating antibodies that are isolated directly from convalescent plasma or serum proteins (Figure 2). It derives sequence information of the most dominant antibodies, thereby capturing a useful picture of the humoral immune response to inform therapeutics, diagnostics, and reagent development.
Furthermore, since only serum antibodies are required, polyclonal antibody sequencing avoids culling of the production animal or invasive medical procedures during clinical testing.
De Novo Polyclonal Antibody Sequencing Service at Rapid Novor
Rapid Novor is the world’s first team to sequence antibodies directly from a polyclonal protein sample without cell or nucleic material input. We have accomplished many successful cases, including the recent report of the de novo sequencing of monoclonal antibodies from a goat polyclonal protein mixture. The antibody sequences decoded by us have enabled the recombinant generation of highly functional antibodies, effectively eliminating the batch-to-batch variability issue.
With proven ability to ensure reproducibility of research reagents and great potential for the development of therapeutics and diagnostics, applications and impacts of polyclonal antibody sequencing will be limitless. Reach out to our scientists for inquiries about polyclonal antibody sequencing technology and our antibody discovery services.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics


