Anti-Idiotype: Test Then Express – Functional Pre-Selection Accelerates Antibody Discovery
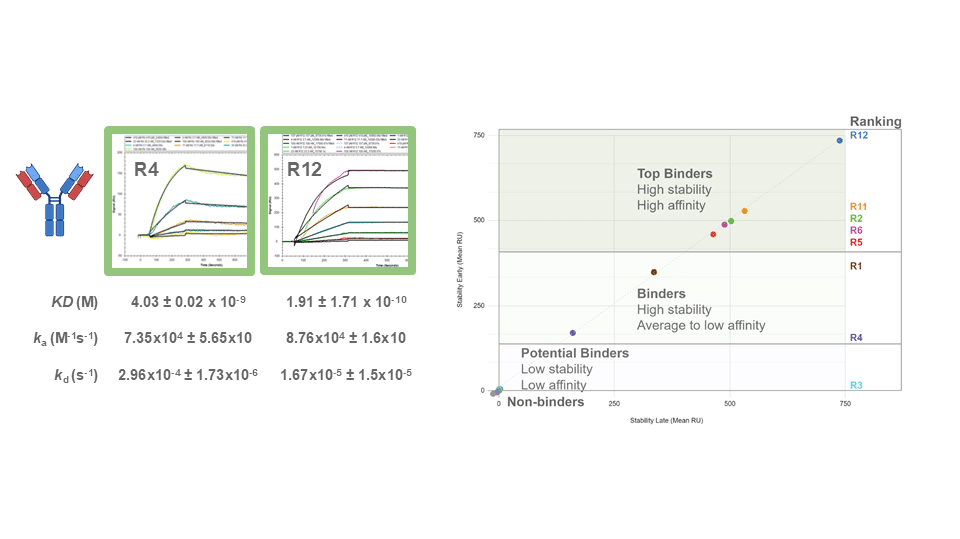
Webinar: July 22, 2025 | 11:00AM EST In this webinar, you will learn: How to efficiently filter out non-specific binders early on to focus only on antibodies with true functional potential. Strategies to isolate highly specific anti-idiotype antibodies that avoid cross-reactivity with natural IgGs. The benefits of combining advanced enrichment techniques [...]