 Written by Yuning Wang, PhD
Written by Yuning Wang, PhD
September 8, 2021
Introduction
In 1892, the administration of polyclonal antibodies (pAb) for therapeutic use was developed to treat tetanus and diphtheria1. Almost a century later, in 1986, the FDA approved the first monoclonal antibody (mAb) drug, muromonab-CD3, for patients undergoing organ transplantation to help reduce the risk of transplant rejection2.
The transition from polyclonal antibody drugs to a more targeted monoclonal approach was made possible through a series of scientific and technological advancements; the most notable of which is the hybridoma technique developed by Köhler and Milstein, which allowed the generation of pure antibodies at scale.
Today, both polyclonal antibody drugs and monoclonal antibody drugs represent important therapeutic tools in a flourishing pharmaceutical field. Monoclonal antibodies have become the main class of novel drugs being developed, as well as the best-selling drugs in the pharmaceutical market. Their success can be attributed to their higher efficacy, reassuring safety profiles, and diverse array of applications; helping to treat a multitude of diseases including cancers, autoimmune, cardiovascular, metabolic, and infectious diseases.
Monoclonal Antibody Drugs
Therapeutic antibodies commonly fall under two major classes: monoclonal and polyclonal antibody drugs. Monoclonal antibodies are commonly produced by challenging an animal with an immunogenic agent, then isolating B-lymphocytes from the animal’s spleen (Figure 1 – top panel). These B-cells are then fused with a myeloma cell line to generate an immortalized cell line, the most common of which are known as a hybridoma, that is capable of producing near-limitless antibodies.
As a single parent B-cell produces these antibodies, the latter immortalized cell lines are homogeneous and only recognize a single epitope per antigen. As such, monoclonal antibodies are prized for having low batch-to-batch variability and low cross-reactivity in contrast to their polyclonal counterparts. In addition, their remarkable specificity makes monoclonal antibody drugs excellent therapeutic agents. To date, 100 mAb drugs have been approved by the FDA with hundreds more in clinical development.
Polyclonal Antibody Drugs
As with monoclonal antibody drugs, the generation of polyclonal antibody drugs begins by inoculating an animal with a specific antigen to elicit an immune response (Figure 1 – bottom panel). Depending on the polyclonal antibody drug that is being produced, the antigen needs to be carefully selected.
Antigens can include a wide variety of substances such as peptides, viruses and cells. In accordance with a natural immune response, a heterogeneous mixture of antibodies is produced, which is then extracted from the animal’s serum. These polyclonal antibodies are capable of binding multiple epitopes on a single antigen and, for this reason, they have an increased overall affinity against the antigen of interest compared to monoclonal antibody drugs.
As a result of the inherent variation within polyclonal antibody drugs, they display greater tolerance to minor changes in the target antigen, which might occur through polymorphisms and glycosylation. Moreover, polyclonal antibody drugs are favored for their shorter production times and lower production costs compared to their monoclonal counterparts, although they possess greater batch-to-batch variability.
Polyclonal antibody drugs were once administered to treat pneumonia, scarlet fever, and meningococcal meningitis3, however, the introduction and rapid development of vaccines, antibiotics, and use of monoclonal antibody therapies — in conjunction with the inherent limitations of polyclonal antibodies — have led to decreased usage of polyclonal antibody drugs.
Nonetheless, a number of animal-derived polyclonal antibody drugs are still marketed, including immunosuppressive therapies and antivenoms3. The latter is particularly relevant as passive immunization or polyclonal antibody therapy remains the main and sometimes only treatment. Moreover, human-derived polyclonal antibody drugs are available as prophylactics against tetanus and cytomegalovirus disease, as well as for passive protection to hepatitis B, to name a few applications3.
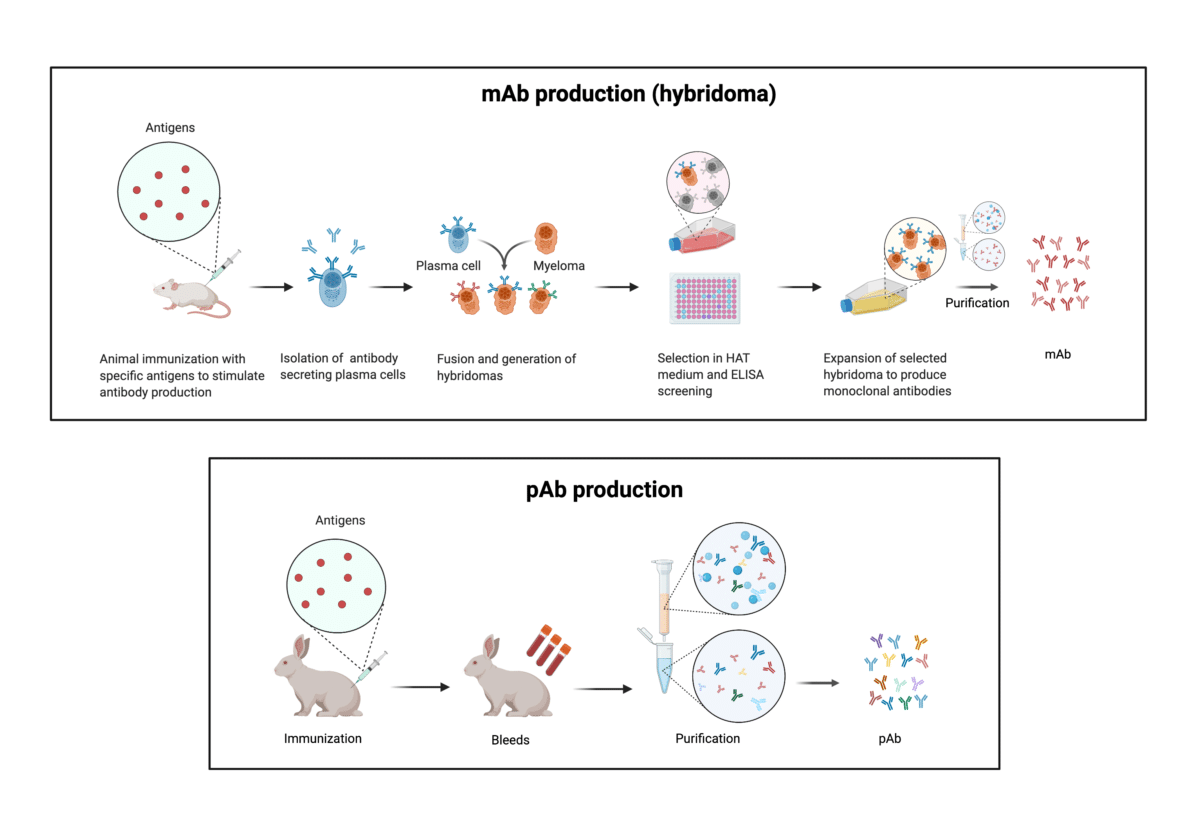
Figure 1. Illustration of the stages in the production of monoclonal antibodies through hybridomas (top panel) and polyclonal antibodies (bottom panel).
Antibody and Protein Sequencing Service
Antibodies are a versatile and ubiquitous tool in the biotech, pharmaceutical and life science industries. While most applications use a single monoclonal antibody in a given context, polyclonal antibodies have been widely used to tackle difficult targets. However, due to their batch-to-batch variation, pAb-based drug development requires culling many production animals; in addition, polyclonal antibodies have been and continue to be extremely challenging to characterize, which limits the identification of strong candidate leads from pAb pools. These challenges have greatly limited the therapeutic use of polyclonal antibody drugs.
To offer a better alternative and usher in a new era in polyclonal antibody discovery, Rapid Novor developed antibody discovery service REpAb®. REpAb® is a next-generation protein sequencing-powered antibody discovery platform that enables users to sequence antibodies directly from serum or a polyclonal mixture. This enables recombinant antibody expression of select clones which, together, can effectively recapitulate the activity of pAb. REpAb®’s breakthrough technology empowers scientists to capitalize on the robust binding characteristics of polyclonal antibodies with the stability and reproducibility of recombinant mAbs. Moreover, REpAb® gives information about post-translational modifications, reduces the use of animals, and increases the production scale and reproducibility of antibody products through recombinant expression.
References
- Pucca, M. B. et al. History of Envenoming Therapy and Current Perspectives. Frontiers in Immunology 10, 1598 (2019).
- Lu, R.-M. et al. Development of therapeutic antibodies for the treatment of diseases. Journal of Biomedical Science 27, 1 (2020).
- Newcombe, C. & Newcombe, A. R. Antibody production: Polyclonal-derived biotherapeutics. Journal of Chromatography B 848, 2–7 (2007).
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

