What are Recombinant Antibodies?
Recombinant antibodies are artificially synthesized antibodies. Recombinant antibodies are generated from expression systems (e.g., E.coli, yeast, mammalian cell lines) via transfection with two separate plasmids encoding the amino acid sequences for the light and heavy chains, respectively (Figure 1). In order to recombinantly produce mAbs, the amino acid sequence of the light and heavy chains must be known. There are many ways to obtain the sequence of an antibody.
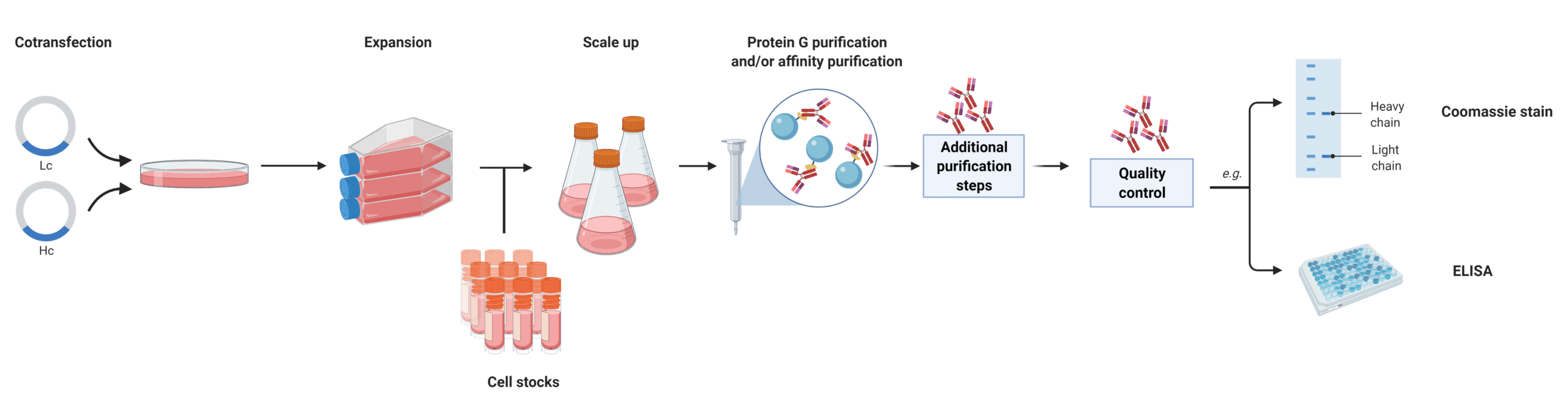
Figure 1. Recombinant antibody production in mammalian cell lines.
What is the Difference Between Recombinant and Traditional Antibodies?
The main difference between the two is that traditional antibodies are created by biological systems without control over the sequence, whereas recombinant antibodies are created according to a desired sequence. Briefly, traditional antibody production involves inoculating animals with a target antigen and an adjuvant to incite a humoral immune response. Four to six weeks post immunization, bleeds are collected, or the spleen is harvested from the production animal.
At this point, antibodies can be purified from sera in various ways (e.g., protein A/G, and/or against a target antigen) and used as a polyclonal mixture for immunoreagent purposes, or cells can be isolated from the bleeds or spleen to generate hybridomas – a B-cell fused to a myeloma cell – or other fusion cell lines. To date, hybridomas remain the most commonly used method of producing antibodies from single hybridoma clones (i.e., monoclonal antibodies).
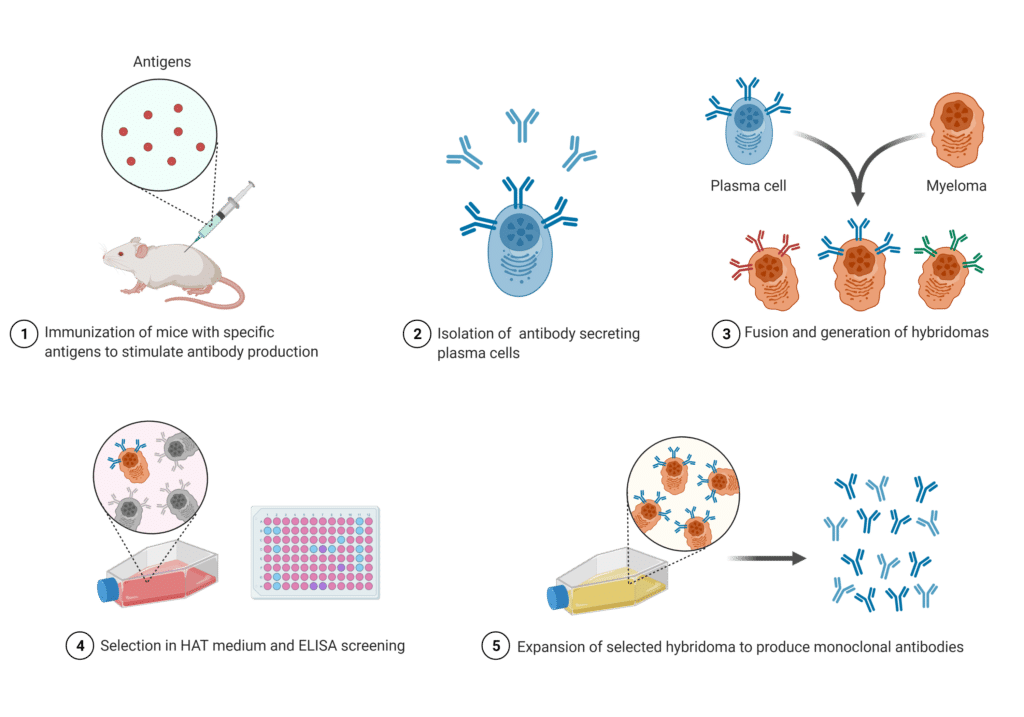
Figure 2. Schematic of the traditional hybridoma technology used to produce monoclonal antibodies.
Why are Recombinant Antibodies Important?
Because each hybridoma cell line is generated from a single B-cell clone, it is expected to output copious amounts of a single antibody clone – thus the term monoclonal antibody (mAb). Nevertheless, this isn’t always the case. Additional chains and other cell instability artifacts can have an impact on each antibody batch. Aside from reproducibility issues, traditional antibodies may also lack sufficient specificity and affinity due to these artifacts. But, they are very easy to produce.
To circumvent these issues, recombinant technology, the ability to produce proteins through biological systems, has been applied to the production of monoclonal antibodies. Because the sequence is known, quality control is strengthened, reproducibility increased, and research insured as losing a cell line does not have to jeopardize an important mAb’s manufacturing.
Types of Recombinant Antibodies
To date, antibodies are generated by first inciting an immune response. However, post-immunization, depending on how an antibody will be utilized, traditional discovery and manufacturing may vary. For instance, because they need to comply with clinical efficacy and safety measures, antibody therapeutics are bound to more stringent standards than immunoreagents.
While in research settings, and immunoreagent production, hybridoma technology is the most commonly used approach, in the therapeutic industry, antibodies are produced via sequencing-guided methods to ensure that the production of an antibody therapeutic can be successfully replicated indefinitely. Recombinant antibodies therefore can be found in a variety of applications: as research reagents, as therapeutics, and as diagnostic immunoreagents, and they can be made from any animal, isotype, or subtype.
How are Recombinant Antibody Sequences Obtained?
De Novo Design of Antibodies
Despite recombinant antibodies being made from a sequence, there is yet no widespread method of artificially generating antibodies without first relying on a biological system. Some research has been published on the use of artificial intelligence to design and engineer antibodies de novo1 by harnessing knowledge from antibody sequences, structure and physicochemical properties.
However, the technology remains inaccessible to most of the life sciences research community. Aside from de novo design, there are many other ways to obtain the sequence of an antibody for recombinant antibody expression. Two methods that are used to obtain the sequence of an antibody are DNA and Protein Sequencing.
Nucleotide Sequencing
There are benefits to both, and the two techniques are complementary to each other. Nucleotide sequencing methodologies include RNA and/or DNA sequencing (e.g., next generation sequencing) of hybridomas, circulating B-cells obtained from peripheral mononuclear blood cells, and/or B-cells from the spleen of lab animals.
Protein Sequencing
With next generation protein sequencing-guided antibody discovery, countless researchers benefit from information on the primary structure, including post-translational modifications (PTMs) that can impact yield, affinity, specificity, and half-life. Recently, Rapid Novor’s NGSP technology (REmAb® antibody sequencing service) was cited as part of a framework to generate recombinant antibodies2. We have also become the first team in the world to generate recombinant antibodies directly from serum using only proteomics through our REpAb® antibody discovery service.
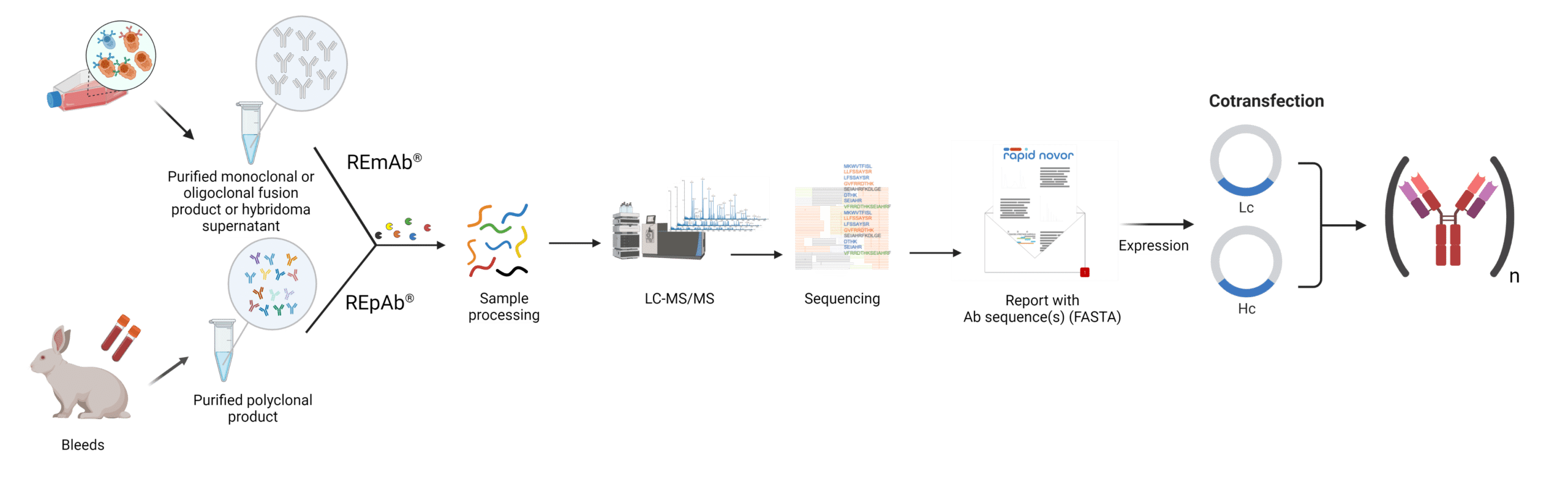
Figure 3. Workflow of two next generation protein sequencing approaches tailored to antibodies to obtain the light and heavy chain sequences for the recombinant expression of monoclonal or polyclonal antibodies.
Why Next Generation Protein Sequencing is Ideally Suited to Recombinant Antibody Development
The latter represents the first instance goat polyclonal antibodies have been sequenced and recapitulated in recombinant form. This carries special significance for veterinary applications, where immunoreagents and valuable biomarkers from different species are on demand but lacking. But it also represents an opportunity to generate better standards for immunoassays such as those used for anti-drug antibody testing.
Most importantly, next generation protein sequencing can and has been used to develop robust antibody therapeutic cocktails that will likely be needed for emerging pathogen outbreaks in the future3,4. Typically, cellular input is needed to extract this information, but with REpAb®, recombinant antibody cocktails can now more faithfully capture the natural immune response. Sequence information obtained from next generation protein sequencing already contains critical information on PTMs that contribute to structure, function, and stability. This information can be used to guide rational design and further study of monoclonal therapeutics and diagnostics5,6 for clinical safety, efficacy and significance.
Protein Sequencing Service
The following upcoming webinar: “Recombinant Therapeutic pAbs Are Now Possible” provides an overview of the aforementioned breakthrough. To keep up to date with next generation protein sequencing and other relevant research, you may sign up to our newsletter. Finally, for inquiries about next generation protein sequencing and/or proteomics technology and services, reach out to our scientists.
References
- Pan, X., and Kortemme, T. (2021) Recent advances in de novo protein design: Principles, methods, and applications. J Biol Chem 296: 100558, doi.org/10.1016/j.jbc.2021.100558
- DeLuca et al. (2021) Generation and diversification of recombinant monoclonal antibodies for studying mitosis. bioRxiv 11 Sep 2021,doi.org/10.1101/2021.09.10.455288
- Dang et al. (2021) Broadly neutralizing antibody cocktails targeting Nipah virus and Hendra virus fusion glycoproteins. Nat Structural & Mol Bio 28: 426-434 doi.org/10.1038/s41594-021-00584-8
- Janssen Biotech, Inc. (2021) United States Patent Application 20210032338 MATERIALS AND METHODS FOR MODULATING T CELL MEDIATED IMMUNITY.
- Nešić, D. et al. (2020) Cryo-Electron Microscopy Structure of the αIIbβ3-Abciximab Complex. Arteriosclerosis, Thrombosis, and Vascular Biology 40: 624-637, doi.org/10.1161/ATVBAHA.119.313671
- Wang et al. (2021) First Immunoassay for Measuring Isoaspartate in Human Serum Albumin. Molecules 26(21): 6709, doi.org/10.3390/molecules26216709
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

 Written by María Gerpe, PhD
Written by María Gerpe, PhD