 Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: June 10, 2024
Introduction
Recombinant antibody cocktails are mixtures of synthetic antibodies produced in vitro using genetically engineered recombinant expression systems. Designed to mimic natural polyclonal antibodies, they offer enhanced specificity, increased stability, and scalability. Unlike hybridoma-derived monoclonal antibodies, recombinant antibodies maintain consistent specificity and target affinity. Consequently, recombinant antibody cocktails are widely used in research and diagnostics, significantly benefiting biotechnology and medicine.
Advantages of Recombinant Antibody Cocktails
Polyclonal antibodies (pAbs) are used to overcome the limitations of monoclonal antibodies (mAbs), especially in targeting antigens with multiple epitopes or high mutation rates. This was evident during the COVID-19 pandemic when the mAb bamlanivimab was revoked due to variant escape. Similarly, cancer cells can mutate to evade single mAb therapies. Unlike mAbs, pAbs recognize multiple epitopes, improving target neutralization. However, plasma-derived pAbs often suffer from high cross-reactivity, contaminants, limited supply, and batch-to-batch variation. Studies have shown that plasma derived pAbs can contain infectious agents, clotting factors, and cause allergic reactions. Moreover, their production is restricted by the immune repertoire of one donor host leading to low target neutralization and limited production capacity due to host culling or loss.
As a solution, recombinant antibody cocktails can be used to capture a more diverse antibody repertoire than a single recombinant antibody, while also addressing the production problems of pAbs (Figure 1). For instance, after single mAb and pAb testing failed, a recombinant antibody cocktail successfully cured symptomatic rabies in mice by preventing viral escape and increasing variant coverage. Given that antibody cocktails are made of antibodies with known sequences that are highly specific to a single antigen, they are less cross-reactive than pAbs and highly reliable and reproducible. Additionally, when compared to monoclonal antibodies synthesized using traditional hybridoma techniques, recombinant antibody cocktails allow for animal-free manufacturing, high lot-to-lot consistency, high manufacturing capacity, low production time, and advanced engineering capabilities.
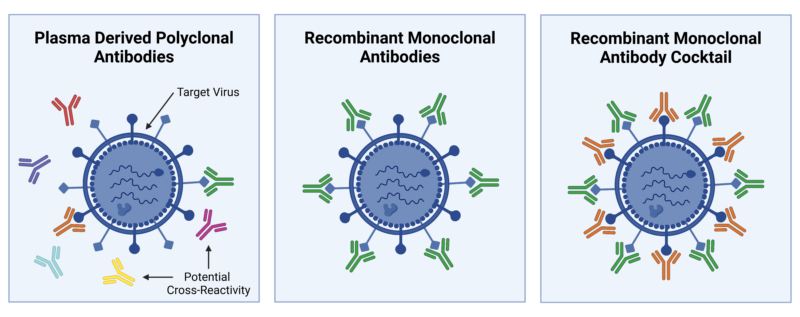
Figure 1: Target Neutralization Capabilities of Plasma Derived Polyclonal Antibodies, Recombinant Monoclonal Antibodies, and Recombinant Monoclonal Antibody Cocktail.
Recombinant Antibody Cocktail Formats
Recombinant antibody cocktails can be generated in various formats depending on the application (Figure 2). They can be produced as full-length mAbs, antibody fragments, bispecific antibodies, antibody fusion proteins, or nanobodies. For instance, researchers at the University of Washington developed a Fab cocktail that showed potent neutralizing capabilities against henipaviruses (HNVs) which currently do not have any approved therapeutics or vaccines to combat the viruses in humans. A cocktail of scFv fragments targeting different epitopes on the HIV-1 envelope glycoprotein has significantly improved therapeutic potency. Additionally, bispecific antibody cocktails like Teclistamab and Talquetamab treat multiple myeloma by engaging T cells against different myeloma-associated antigens.
Regardless of the chosen format, these cocktails can be engineered to recognize different antigenic variants, such as anti-Rabies virus antibody cocktails, or different epitopes, like those for Ebola virus and HER-2, enhancing target neutralization. For research, recombinant antibodies can be conjugated with molecules like biotin, enzymes, and fluorochromes, and for therapeutic use, they can be conjugated with drugs.
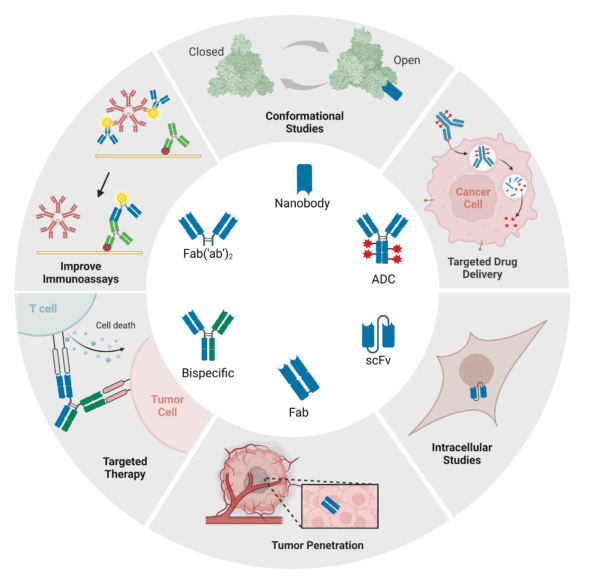
Figure 2: Recombinant Antibody Formats and Associated Applications.
Engineering Recombinant Antibody Cocktails
Engineering antibody cocktails requires careful consideration of various factors to ensure efficacy, safety, and manufacturability. Here are key considerations:
Target Diversity
When engineering antibody cocktails, it is important to select targets that do not interfere with each other’s epitopes and have synergistic effects, such as different antigens on the same target or distinct pathways in disease progression. When designing antibody cocktails, use HDX-MS epitope mapping to find non-overlapping epitopes and measure potency and synergy by epitope binning with surface plasmon resonance analysis (SPR). The Fab regions can also be engineered to bind otherwise inaccessible targets, such as single amino acids, specific post-translational modifications (PTMs), and tight protein domains.
Affinity, Specificity, Half-Life
When designing an antibody cocktail, each antibody in the cocktail should be optimized for affinity and specificity to decrease off-target effects. The potency can be enhanced through Fc glycoengineering and mutations to improve effector functions and serum half-life. Complementary determining regions (CDRs) can also be modified to optimize antigen binding, increase stability and half-life with albumin fusion, and improve diffusion with cell-penetrating peptides (CPPs) fusion. Finally, engineering the Fc region can extend the half-life of the antibodies to increase their therapeutic potency, reducing the need for frequent administration and easing the burden on biopharmaceutical production.
Effector Functions
Engineering the Fc region to induce a specific immune response can reduce or eliminate toxicity when used in biotherapeutics. Depending on the therapeutic goal, this could mean engineering the Fc regions to trigger many different Fc-mediated effector functions such as antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC). Ensure that the combination provides a synergistic effect greater than the sum of its parts. This might involve complementary mechanisms of action, such as blocking different pathways or engaging multiple immune cell types.
Safety
Minimize immunogenicity risk by ensuring the engineered recombinant antibody sequences do not trigger unwanted immune responses. Conduct thorough tests to evaluate potential toxicities, such as cytokine release syndrome. When designing the cocktail, consider unforeseen integrations between the antibodies in the cocktail. To analyze antibody cocktails, various techniques can be used to assess structural integrity, stability, and interactions. Use techniques like circular dichroism (CD) for protein structure evaluation, differential scanning calorimetry (DSC) for conformational stability, and dynamic light scattering (DLS) and static light scattering (SLS) for detecting cross-interactions and aggregation.
Overall, developing and validating antibody cocktails is more complex than individual mAbs. Whether used in biotherapeutics or as standard lab reagents, engineered antibody cocktails ensure standardized safety and efficacy.
Producing Recombinant Antibody Cocktails
Generating a recombinant antibody cocktail involves several steps, from selecting the target antigens to producing and purifying the antibodies. Here’s a detailed overview of the process (Figure 3):
- Antibody Production: Select a target antigen based on experimental objectives and immunize an animal to trigger an immune response. This generates pAbs specific to the antigen. Extract pAbs by collecting blood and purifying the serum using protein A/G, an antigen-affinity purification column, and any negative selection purification desired.
- Select Antibodies: Test the resulting pAbs using techniques such HDX-MS epitope mapping and surface plasmon resonance analysis (SPR) to select the pAbs to proceed with.
- De novo sequencing: Full antibody sequences are generated using de novo antibody sequencing mass spectrometry and machine-learning (ML)-based bioinformatics.
- Plasmid Engineering: Use sequence data from REpAb de novo antibody sequencing to create synthetic genes for the heavy and light chains. Bioplanning parameters can be used to engineer the antibodies to have desired qualities or to select against non-desired qualities using the engineering methods mentioned above. Insert final engineered antibody sequences into appropriate plasmid expression vectors.
- Recombinant Cell Line Transfection: Transfect engineered plasmid expression vectors into an appropriate recombinant expression system. Choose an appropriate cell line based on the intended application (See how to choose a recombinant cell line here).
- Produce Recombinant Antibodies: Purify the antibodies from the culture supernatant using protein A/G affinity chromatography, ion exchange chromatography, or other methods.
- Antibody Characterization: After purification, the antibody or nanobody can be put through different quality control measures, such as SDS-PAGE, ELISA, and peptide mapping by mass spectrometry.
- Formulation and Testing: Combine the purified and characterized antibodies then perform methods such as DLS and DSC to refine the ratio of antibodies and test the cocktail stability, efficacy, specificity, and safety. Adjust antibody formulation ratios to ensure peak performance.
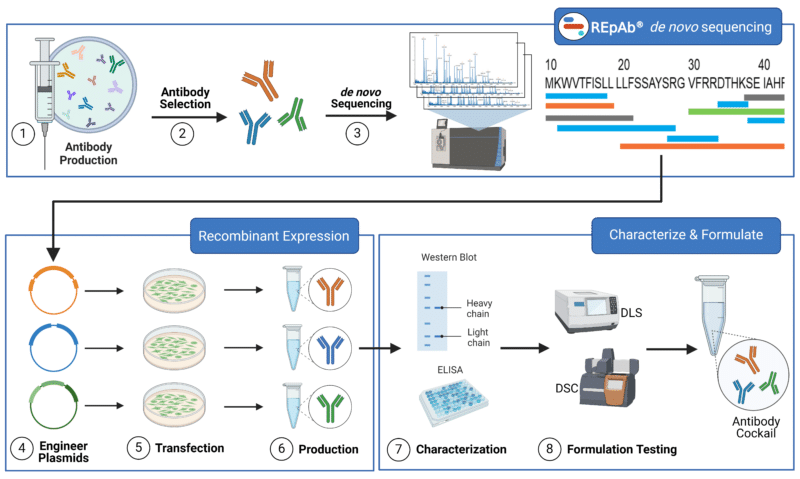
Figure 3: Step-by-step Process to Develop Recombinant Antibody Cocktail.
Recombinant Antibody Cocktail Applications
Since their development, recombinant antibody cocktails have revolutionized various research applications, including western blot (WB), immunohistochemistry (IHC), immunofluorescence (IF), enzyme-linked immunosorbent assay (ELISA), flow cytometry (FC), and numerous other antibody-based assays. These cocktails have notably enhanced sensitivity, specificity, and multiplexing capabilities. For instance, studies indicate that recombinant antibody cocktails can increase ELISA sensitivity by up to 20-fold. They have also improved IHC and FC by enabling multiplexing and co-localization, and Fab cocktails have advanced structural biology assays. Overall, recombinant antibody cocktails have been instrumental in driving scientific research forward and addressing the reproducibility crisis in laboratory research.
Beyond research applications, recombinant antibody cocktails have significantly advanced antibody-based diagnostics and therapeutics. Recent studies demonstrate that these cocktails have markedly improved diagnostics for detecting human T-cell leukemia virus (HTLV) and Group A Streptococcus (GAS) infections. Additionally, during the SARS-CoV-2 pandemic, antibody cocktails have been crucial in enhancing diagnostics and therapeutics by mitigating mutant escape. Furthermore, in cancer therapeutics, antibody cocktails have shown to outperform single antibodies by up to 244-fold in targeted alpha therapy. These examples underscore the profound impact of recombinant antibody cocktails on medical diagnostics and patient outcomes across a range of health conditions.
Generating Antibody Cocktails with De Novo Antibody Sequencing Service
To ensure the production of accurate, reliable, high-quality antibodies that meet specific functional requirements in antibody cocktails, consider the following services:
- Monoclonal antibody sequencing service: Determine the sequence of monoclonal antibodies which can be used to produce recombinant mAbs.
- Antibody Discovery Service: Convert pAbs to mAbs using polyclonal antibody sequencing, identifying key mAbs in a pAb mixture for recombinant expression and antibody cocktail development.
- Epitope Mapping Service: Allows for antibody epitope mapping to ensure the antibodies selected for the cocktail target biologically relevant and non-overlapping epitopes.
- SPR Service: Utilize surface plasmon resonance analysis to screen, select, and profile the antibodies within the cocktail via epitope binning to measure synergy and potency.
- Recombinant Antibody Expression Service: Produce your antibody cocktail with the fastest and highest protein quality protein production.
- Antibody Characterization Services: Identify post-translational modifications (PTMs), sequence variants, and disulfide linkages early in the antibody cocktail production pipeline.
To explore how Rapid Novor can enhance your antibody cocktail engineering, production, and quality control processes contact our scientists today!
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

