 Written by:
Written by:
Vanessa Yoon Calvelo, PhD
Updated: January 19, 2023
(Published: June 2, 2022)
The Importance of Post-Translational Modifications (PTMs)
Post-translational modifications (PTMs) are processing events typically driven by enzymatic reactions that alter the chemical properties of amino acids, thus influencing the structure and dynamics of the protein. PTMs can occur at one or multiple sites, at any point in time, upon protein synthesis and release from the ribosome. Some modifications occur shortly after translation to mediate protein folding or to direct protein localization. Other modifications occur during the stepwise mechanism of protein maturation, which influences the biological activity of the protein. Additionally, there are certain modifications that tag the protein for degradation.
What are the Consequences of PTMs?
PTMs can alter a myriad of protein behaviours and characteristics including enzyme function and assembly, protein lifespan, protein-protein interactions, molecular trafficking, receptor activation, protein solubility, protein folding, and protein localization. As a result, they often influence biological processes, such as signal transduction, gene expression regulation, gene activation, DNA repair, and cell cycle control. They also have a hand in various diseases – analysis of PTMs is particularly important for the study of cardiovascular disease, cancer, diabetes, and neurodegenerative diseases. For example, PTMs in the amyloid β peptide and the τ protein are biomarkers for several neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and multiple sclerosis (MS).
Types of PTMs
There are more than 400 different types of PTMs that affect many aspects of protein structure and function. Generally, PTMs can be reversible through covalent modifications; however, there are irreversible modifications that proceed in one direction (ie. proteolytic cleavage). The top five most studied PTMs include:
- Phosphorylation – addition of a phosphate typically to Ser, Thr, Tyr residues, catalyzed by kinases. Phosphorylation is involved in processes such as replication, transcription, stress response, cell movement, cell metabolism, apoptosis, and immunological responsiveness. Defects in the phosphorylation pathway are implicated in cancer, Alzheimer’s disease, Parkinson’s disease, and cardiovascular disease.
- Acetylation – addition of an acetyl group typically to the N-terminus of a protein or Lys residues, catalyzed by acetyltransferases. Acetylation is involved in processes such as chromatin stability, protein-protein interaction, cell cycle control, cell metabolism, and nuclear transport. Defects in acetylation are implicated in cancer, aging, immune disorders, Huntingotn’s disease, Parkinson’s disease, and cardiovascular disease.
- Ubiquitylation – addition of ubiquitin typically to Lys residues (however, this is a versatile PTM that can occur on all 20 amino acids), catalyzed by the ubiquitin-activating, ubiquitin-conjugating, and ubiquitin ligase enzyme complex. Ubiquitylation is involved in processes such as proliferation, transcriptional regulation, DNA repair, replication, intracellular trafficking, signal transduction, protein degradation, innate immune signaling, autophagy, and apoptosis. Defects in the ubiquitin pathway are implicated in cancer, metabolic syndromes, inflammatory disorders, diabetes, neurodegenerative diseases.
- Methylation – addition of a methyl group typically to Lys or Arg residues, catalyzed by methyltransferases. Methylation is involved in fine tuning numerous processes, and defects in this PTM are implicated in cancer, Angelman syndrome, and diabetes.
- Glycosylation – addition of sugar groups typically to Ser, Thr, Asn, Trp residues, catalyzed by glycosyltransferases. Glycosylation is involved in processes such as cell adhesion, molecular trafficking, receptor activation, protein solubility, protein folding, signal transduction, and protein degradation. Defects in glycosylation are implicated in cancer, liver cirrhosis, diabetes, HIV infection, Alzheimer’s disease, and atherosclerosis.
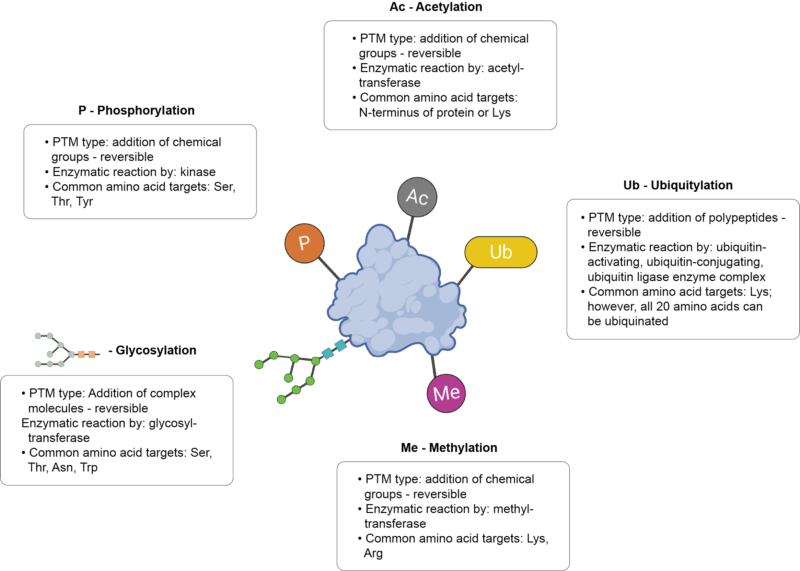
Figure 1. The top five most studied PTMs.
PTMs Increase Microheterogeneity of Antibodies
Considering their vast numbers, PTMs are key mechanisms to increasing proteomic diversity. The human proteome is estimated at >1 million proteins, and the various PTMs add another layer of variability beyond amino acid sequence variation. Sequence variation is notably critical for the structure and function of many types of proteins, including antibodies. Antibody variable domains (Fv) have high sequence variability for antigen-binding specificity and are linked to different constant domains (Fc) for distinct Fc-mediated effector functions. Further adding to this microheterogeneity are the PTMs in these domains that modulate the binding and activity of antibodies. Thus, there has been a shift, from being viewed as hindrances to antibody manufacturing, to exploiting PTMs for the modification and improvement of specific antibody functions.
Types of PTMs in Antibodies
Glycosylation is one of the most frequently observed PTMs in antibodies, and plays a role in regulating many drug side effects including antibody-dependent cellular cytotoxicity and phagocytosis. Other PTMs in antibodies to make note of include: glycation, C-terminal lysine clipping, deamidation, oxidation, cyclization (ie. N-terminal glutamate to pyroglutamate), and disulfide scrambling of the hinge region interchain disulfide bonds (Figure 2).
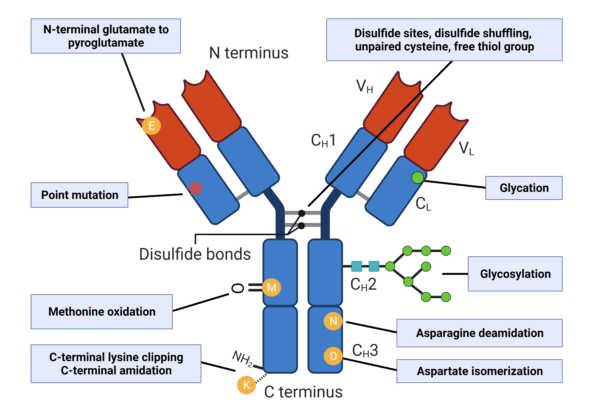
Figure 2. Types of PTMs that increase antibody microheterogeneity.
PTM Analysis by LC-MS
Characterization of PTMs can provide insight into the cellular functions that underly physiological and etiological processes. Some of the challenges to studying PTMs, however, are due to the lack of specificity in detection methods. As proteomic technologies and machine learning algorithms become more advanced and refined (starting with protein sequencing by mass spectrometry), the analysis of post-translationally modified proteins is becoming more accessible. Considering the diversity of PTMs and the report of new PTMs every few years or so, their characterization and manipulation will surely become more routine in the future.
Rapid Novor offers a full suite of antibody characterization services using LC-MS, including PTM analysis, glycan analysis, and disulfide bond analysis.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

