Written by Yuning Wang, PhD
Written by Yuning Wang, PhD
August 25, 2021
Introduction
Monoclonal antibodies (mAbs) are homogenous antibodies that bind to a single epitope on an antigen. Kohler and Milstein generated the first mAbs when they developed hybridoma technology in the 1970s. Because of the specificity, homogeneity and unlimited availability, mAbs are valuable reagents used in a variety of important applications including treatment and diagnosis of diseases1.
Monoclonal Antibodies as Therapeutics
Monoclonal antibodies are the most used immunotherapy method for various diseases such as cancer and infections. With immunotherapy, mAbs’ ability of binding specifically to certain proteins or cells is exploited to boost the immune system against diseased cells.
The first FDA approved mAb drug, Muromonab, was developed to specifically bind to the CD3 receptor on the surface of human T cells to prevent T cell-driven acute rejection in patients with organ transplants1. To date, the FDA has approved 100 mAb drugs that have a wide range of cellular targets such as PD1/PDL1, HER2, and EGFR2.
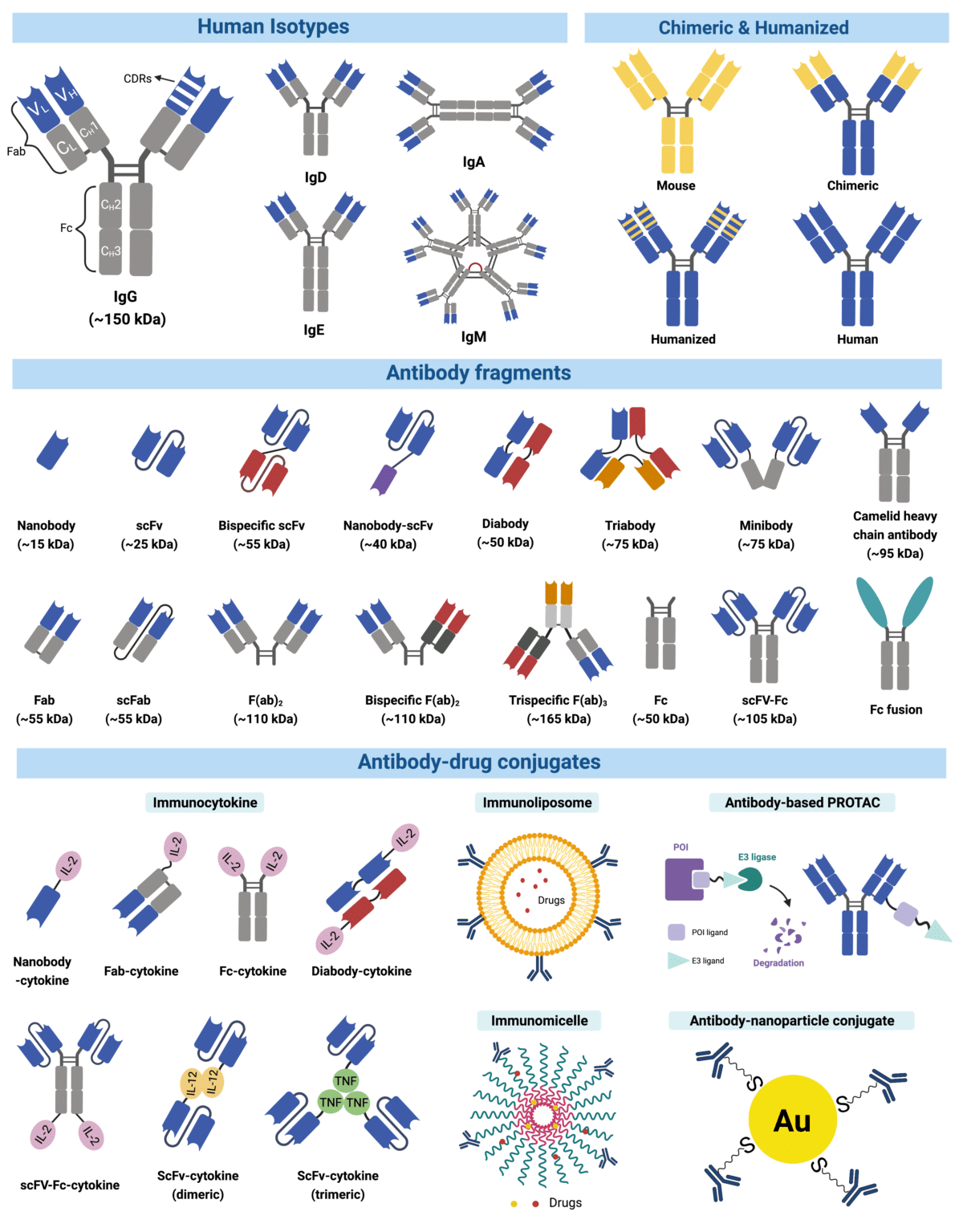
To treat diseases at the clinic, mAbs can remain canonical, or be engineered into different formats (e.g., conjugated, bispecific, fragments mAbs, etc. – see Figure 1)2. Depending on their configuration, mAb drugs work differently per treatment. A typical canonical mAb usually works by binding to an antigen (i.e., on a pathogen or an antigen-presenting cell) to trigger an immune response to destroy the pathogen or cell. Engineered mAbs such as conjugated mAbs are coupled to a small molecule drug or radioactive element3, so that they may deliver these drugs, or elements4, directly to cancer cells, like a trojan horse5. Another type of engineered mAbs – bispecifics – is used as a “bridge” to simultaneously target two cellular receptors on a cancer cell and a cytotoxic T lymphocyte to orchestrate targeted cancer cell death5,6.
Figure 1. Infographic illustrating types of monoclonal antibodies. Therapeutic antibodies are typically engineered off an IgG backbone. Though they used to simply involve chimeras, or humanized antibodies7, monoclonal antibodies now include a range of formats, including proteolysis-targeting chimeras (PROTACs)-conjugated antibodies8, antibody micelles9, antibody-cytokine fusion proteins10, trispecifics and many more4.
Monoclonal Antibodies in Diagnostics
In vitro diagnostics (IVDs) are one of the most used tools to detect diseases, and guide treatment decisions. For many IVD assays – known as immunoassays (Figure 2), mAbs’ binding capacity is exploited to target antigens in colorimetric reactions for detection of pathogens, cellular factors, or other substances11.
- Enzyme-linked Immunosorbent Assay (ELISA) is the most used immunoassay. With ELISAs, technicians screen for the presence and can measure the concentration of an antigen in a liquid sample such as blood and body fluids. There are many types of ELISAs. A commonly used ELISA is the direct ELISA; in direct ELISAs, an antigen is immobilized onto a surface; a mAb linked to a reporter enzyme or fluorochrome is added, followed by a substrate to produce a detectable signal – most commonly a colour change11,12.
- Immunohistochemistry (HIC) is a method primarily used in tissue sections for pathology-based diagnosis of cancer, or infectious diseases. In HIC, enzyme immunolinked or fluorescent dye labelled mAbs bind to target antigens; the binding triggers a reaction that allows the antigen and its distribution in the tissue section to be visualized under a microscope. For example, suspected breast cancer cases can be confirmed by HIC using mAbs against breast cancer biomarkers such as the HER2 receptor protein in tissue sections from a biopsy12.
- Flow cytometry is a laser-based fluidics technology that measures the biochemical and physical characteristics of a population of cells or particles. Most recently, flow cytometry has been used to detect viruses. Traditionally, at the clinic, cells in suspensions are injected into a flow cytometer where they first travel through a sheath to a flow cell. There, a laser is shot at individual cells and a detector records the light reflected and refracted off each cell. Fluorochrome-conjugated antibodies are often used to “stain” the cells for detection of surface biomarkers or receptors. Fluorescent conjugated antibodies bound to the cell surface will emit light based on specific excitation wavelengths coming off the laser. Flow cytometry is commonly exploited to identify many diseases, especially blood-related cancers such as leukemia and lymphoma. Other than confirming presence of pathogens or cancer determinants, flow cytometry is a great indicator of the relative burden of a disease, and an excellent method to characterize cellular populations of the immune system12.
Lateral flow assays and surface plasmon resonance are also used at the clinic, though less commonly to the ones above. Regardless of the method, use of valid mAbs with high affinity and specificity is the key to develop robust and reproducible immunological assays for rapid and accurate diagnosis of diseases12. Learn about how Rapid Novor can help safeguard the development of mAb-based IVDs here.
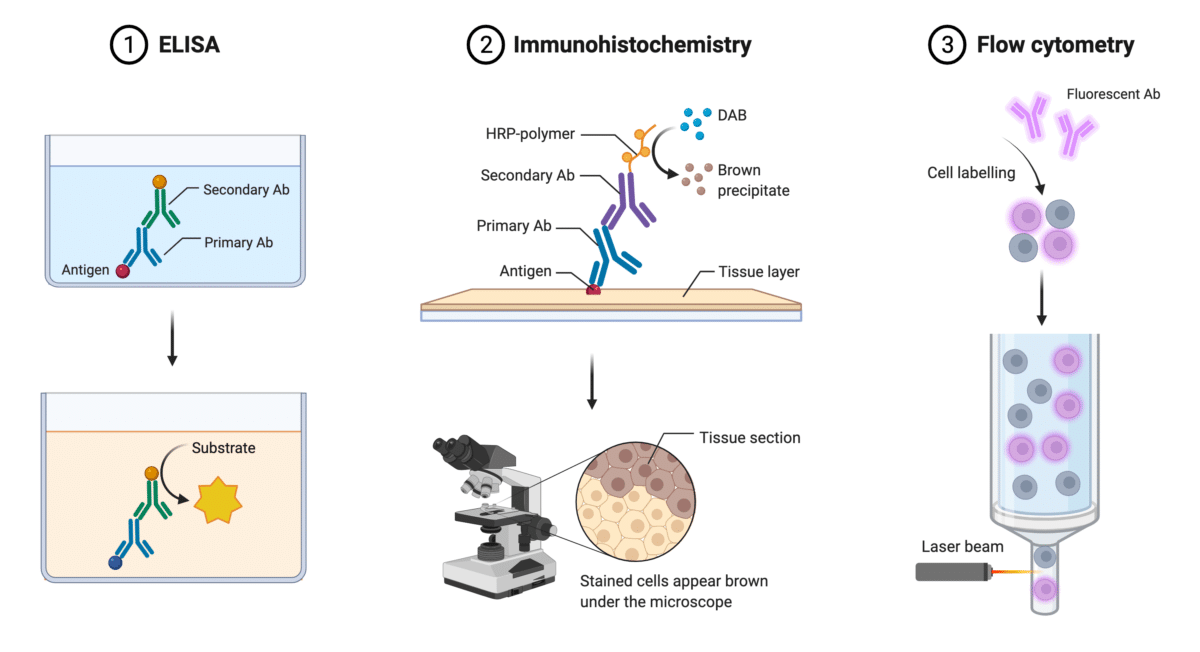
Figure 2. Illustration of common immunoassays: ELISA, Immunohistochemistry, and Flow Cytometry.
How are Monoclonal Antibodies Produced?
There are many ways to manufacture mAbs. A common way is through hybridomas. The process starts by injecting animals with specific antigen that provokes an immune response (Figure 3). Individual B cells producing antibodies that bind to the injected antigen are then isolated from the animal and fused with myeloma cells to produce a hybrid cell line called a hybridoma. By fusing short-lived antibody-producing B cells with immortal myeloma cells, hybridoma cells are expected to provide a never-ending supply of identical mAbs.
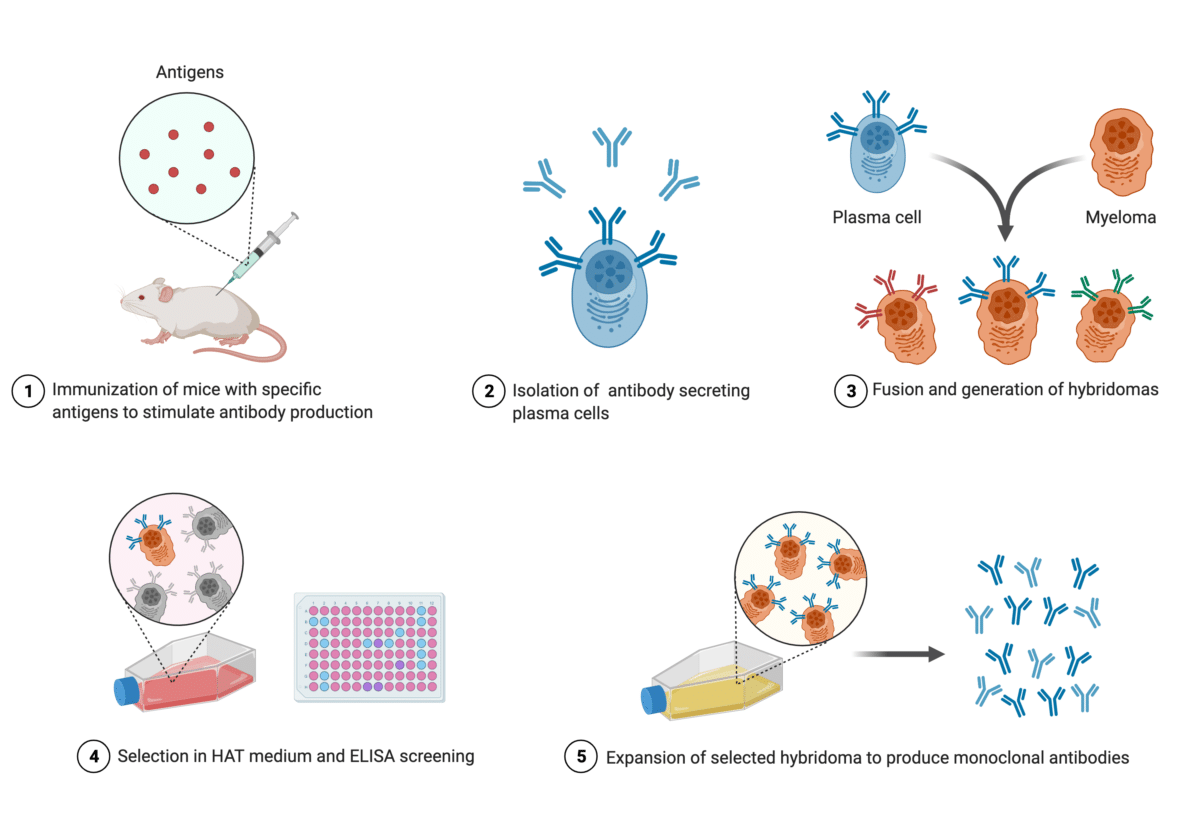
Figure 3. Infographic illustrating the production of mAbs through hybridoma technology
However, plant13 and other mammalian systems14 are available. In contrast to these systems, immortalized B cells (hybridomas) have the “building plans” (genes) of mAbs. As such, expression of antibodies in other cells requires transfection of two constructs (plasmids) bearing the cDNA sequence of the immunoglobulin genes (the light and heavy chain genes). Synthetic antibodies produced in plant or mammalian cells are known as recombinant antibodies (rAbs) (Figure 4). Most mammalian system expressed rAbs are produced in one of these two cell lines: Chinese hamster ovary (CHO) and human epithelial kidney (HEK) 293.
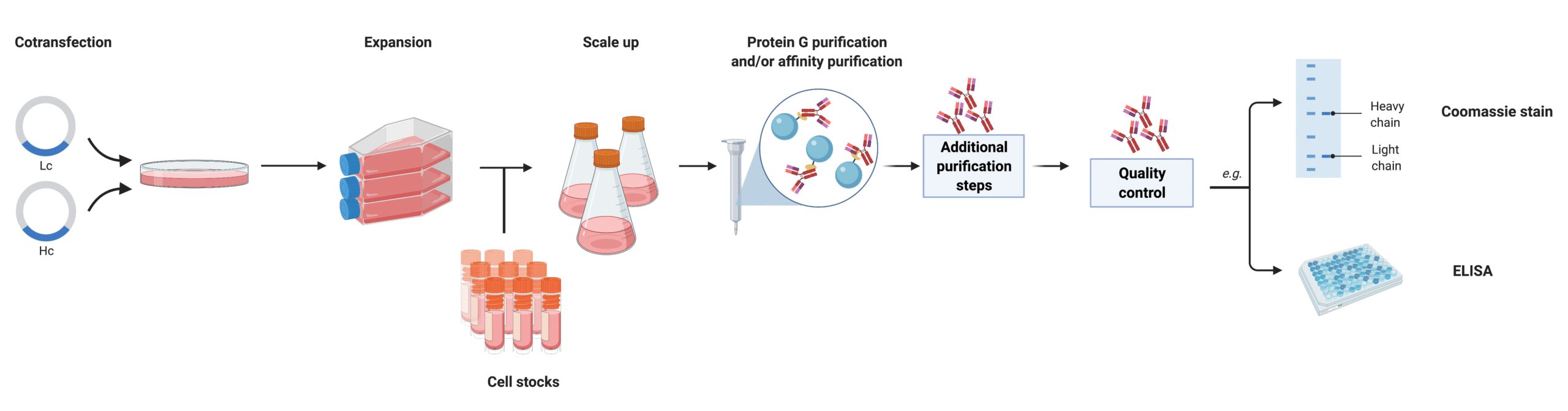
Figure 4. Infographic illustrating recombinant mAb production in mammalian cells.
How are Monoclonal Antibodies Discovered and Developed?
To discover antibodies against a target protein, animals are often inoculated with the target and an adjuvant in order to elicit a natural immune response from the animal and extract active antibodies and biological information for further study. From here on, different processes are used to select the best antibodies for research, medical, or commercial purposes (Figure 5). These methods include hybridoma development, single B-cell sequencing, and display, to name the prominent ones.
Traditional Antibody Discovery Methods
- In hybridoma development, splenocytes containing plasma cells are fused with myeloma cells or immortalized with an oncogenic vector to establish a cell line that will produce antibodies in the long-term15.
- In single B-cell sequencing, isolated peripheral blood mononuclear cells are cell sorted using flow cytometry to sequence individual B lymphocytes’ DNA or RNA using next generation sequencing and obtain the immunoglobulin (Ig) genes for recombinant expression in mammalian cell lines like CHO or HEK 293T cells16.
- Finally, for phage display, or other display technologies (bacteria, yeast), B-cells are isolated from plasma to extract the mRNA and perform RT-PCR and other Ig isotype specific PCR to generate a synthetic repertoire displayed by phage, or bacteria, or yeast for affinity maturation in vitro. These libraries can then be “recycled” to screen against other target proteins17.
Antibody Protein Sequencing: a Complementary Approach to Antibody Discovery
REpAb® is Rapid Novor’s antibody discovery service that is rooted in mass spectrometry and supported by Big Data-powered machine learning. REpAb® is easily integrated into already existing antibody discovery pipelines that can utilize nucleic data such as the aforementioned platforms. But it can also be directly employed on affinity purified bleeds from inoculated animals. REpAb® captures the circulating antibody pool already primed against the target and does not require culling of the production animal.
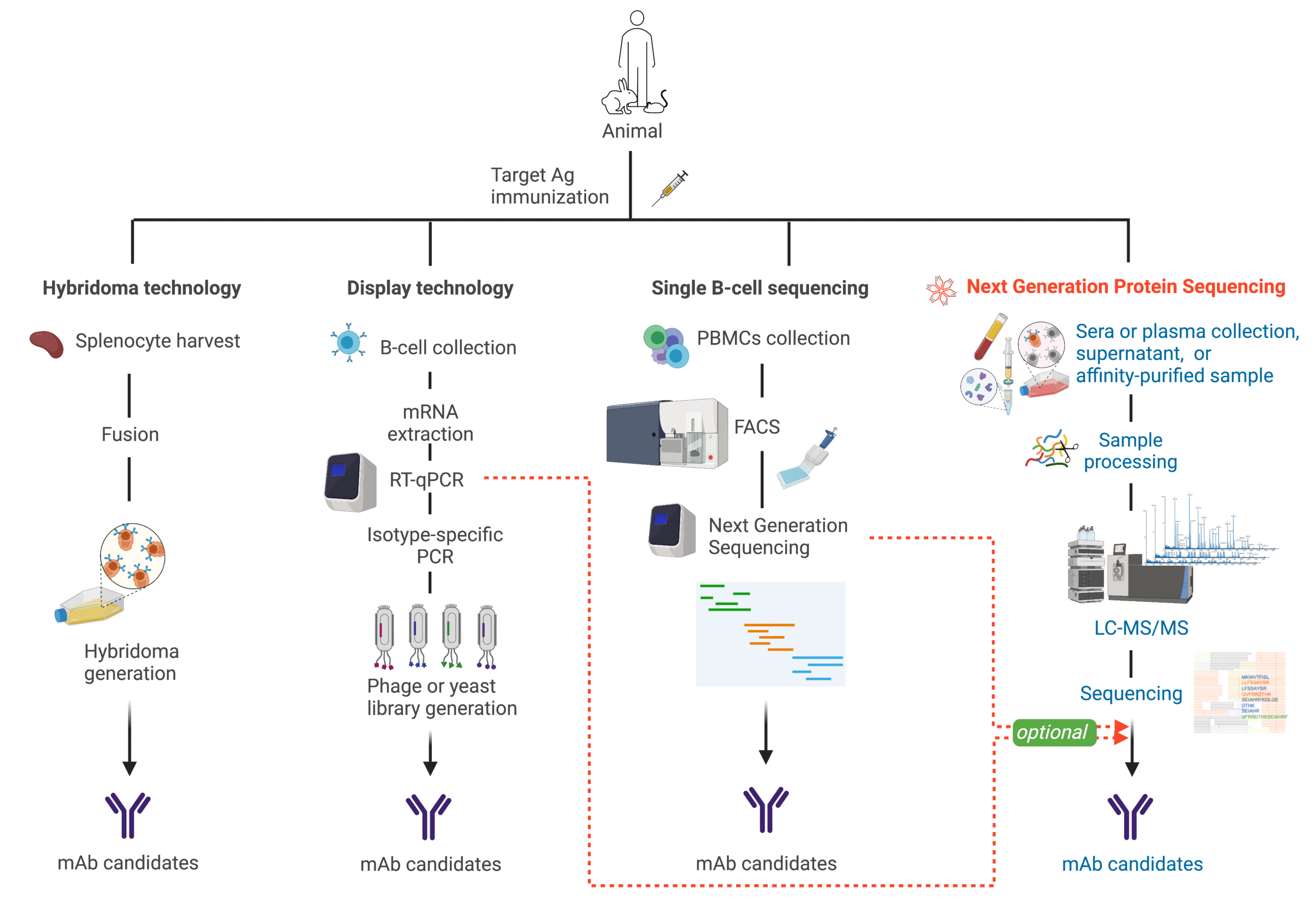
Figure 5. Antibody discovery platforms: Single B-cell sequencing, display, hybridoma, and REpAb®
For simpler samples, such as or samples that have undergone multiple rounds of purification and are considered monoclonal or biclonal, we also offer REmAb® antibody sequencing service, which has been successfully used by our clients in the development of multiple antibodies for therapeutic and research purposes18-20.
References
- Singh, S. et al. Monoclonal Antibodies: A Review. Curr Clin Pharmacol 13, 85-99, doi:10.2174/1574884712666170809124728 (2018).
- Mullard, A. FDA approves 100th monoclonal antibody product. Nat Rev Drug Discov 20, 491-495, doi:10.1038/d41573-021-00079-7 (2021).
- Arruebo, M., Valladares, M. & González-Fernández, Á. Antibody-Conjugated Nanoparticles for Biomedical Applications. Journal of Nanomaterials 2009, 1-24, doi:10.1155/2009/439389 (2009).
- Jazayeri, M. H., Amani, H., Pourfatollah, A. A., Pazoki-Toroudi, H. & Sedighimoghaddam, B. Various methods of gold nanoparticles (GNPs) conjugation to antibodies. Sensing and Bio-Sensing Research 9, 17-22, doi:10.1016/j.sbsr.2016.04.002 (2016).
- Lu, R. M. et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 27, 1, doi:10.1186/s12929-019-0592-z (2020).
- Garfall, A. L. & June, C. H. Trispecific antibodies offer a third way forward for anticancer immunotherapy. Nature 575, 450-451, doi:10.1038/d41586-019-03495-3 (2019).
- Chiu, M. L., Goulet, D. R., Teplyakov, A. & Gilliland, G. L. Antibody Structure and Function: The Basis for Engineering Therapeutics. Antibodies (Basel) 8, doi:10.3390/antib8040055 (2019).
- Cotton, A. D., Nguyen, D. P., Gramespacher, J. A., Seiple, I. B. & Wells, J. A. Development of Antibody-Based PROTACs for the Degradation of the Cell-Surface Immune Checkpoint Protein PD-L1. J Am Chem Soc 143, 593-598, doi:10.1021/jacs.0c10008 (2021).
- Sawant, R. R., Jhaveri, A. M. & Torchilin, V. P. Immunomicelles for advancing personalized therapy. Adv Drug Deliv Rev 64, 1436-1446, doi:10.1016/j.addr.2012.08.003 (2012).
- Kontermann, R. E. Antibody-cytokine fusion proteins. Arch Biochem Biophys 526, 194-205, doi:10.1016/j.abb.2012.03.001 (2012).
- Vashist, S. K. & Luong, J. H. T. in Handbook of Immunoassay Technologies 1-18 (2018).
- Gao, Y., Huang, X., Zhu, Y. & Lv, Z. A brief review of monoclonal antibody technology and its representative applications in immunoassays. J Immunoassay Immunochem 39, 351-364, doi:10.1080/15321819.2018.1515775 (2018).
- Diamos, A. G. et al. High Level Production of Monoclonal Antibodies Using an Optimized Plant Expression System. Front Bioeng Biotechnol 7, 472, doi:10.3389/fbioe.2019.00472 (2019).
- Kunert, R. & Reinhart, D. Advances in recombinant antibody manufacturing. Appl Microbiol Biotechnol 100, 3451-3461, doi:10.1007/s00253-016-7388-9 (2016).
- Zaroff, S. & Tan, G. Hybridoma technology: the preferred method for monoclonal antibody generation for. Biotechniques 67, 90-92, doi:10.2144/btn-2019-0054 (2019).
- Calis, J. J. & Rosenberg, B. R. Characterizing immune repertoires by high throughput sequencing: strategies and applications. Trends Immunol 35, 581-590, doi:10.1016/j.it.2014.09.004 (2014).
- Chan, C. E., Lim, A. P., MacAry, P. A. & Hanson, B. J. The role of phage display in therapeutic antibody discovery. Int Immunol 26, 649-657, doi:10.1093/intimm/dxu082 (2014).
- Nešić, D. et al. Cryo-Electron Microscopy Structure of the αIIbβ3-Abciximab Complex. Arterioscler Thromb Vasc Biol 40, 624-637, doi:10.1161/ATVBAHA.119.313671 (2020).
- Dang, H. V. et al. Broadly neutralizing antibody cocktails targeting Nipah virus and Hendra virus fusion glycoproteins. Nat Struct Mol Biol 28, 426-434, doi:10.1038/s41594-021-00584-8 (2021).
- Dai, D. L. et al. Structural Characterization of Endogenous Tuberous Sclerosis Protein Complex Revealed Potential Polymeric Assembly. Biochemistry 60, 1808-1821, doi:10.1021/acs.biochem.1c00269 (2021).
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics