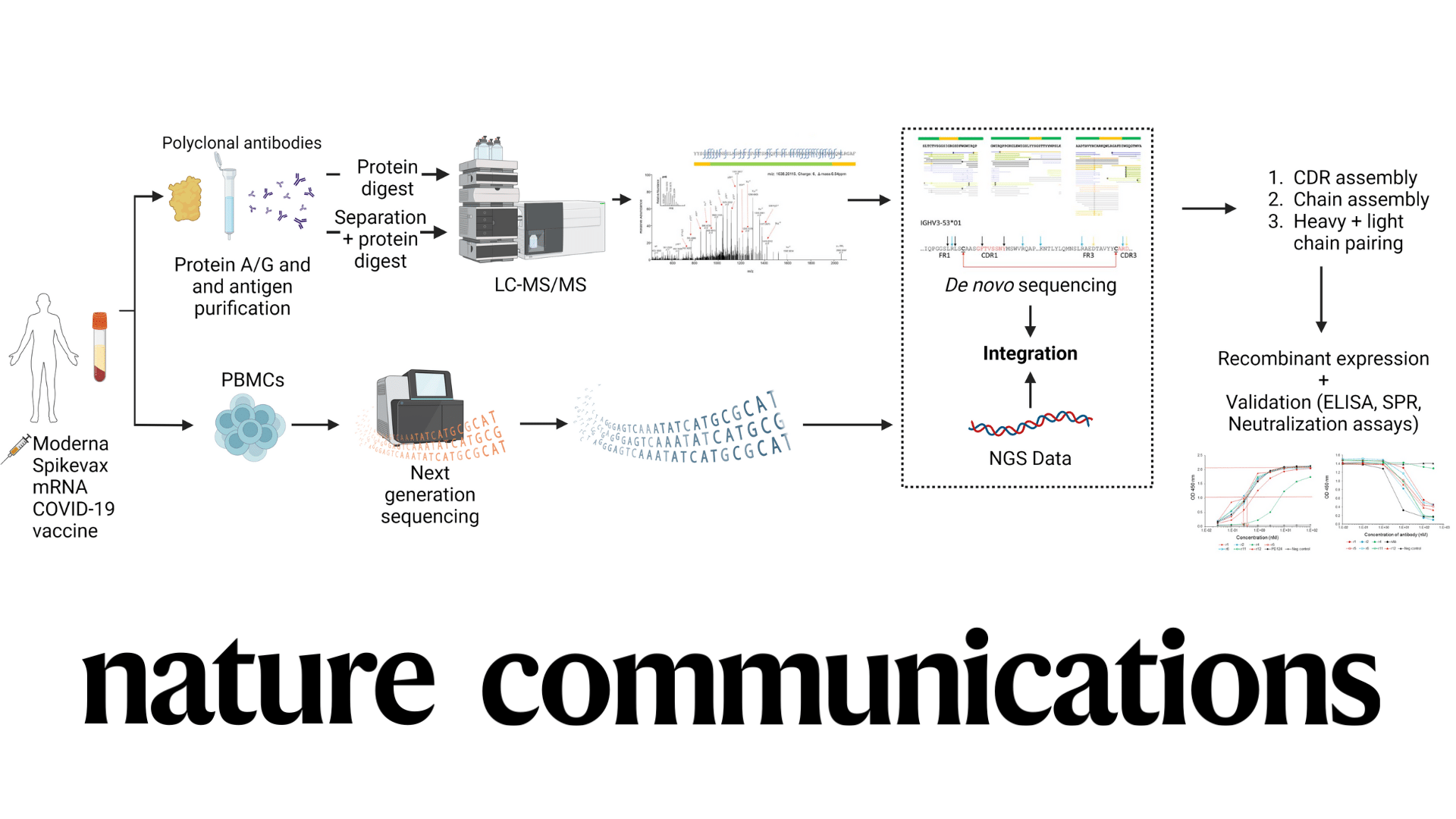
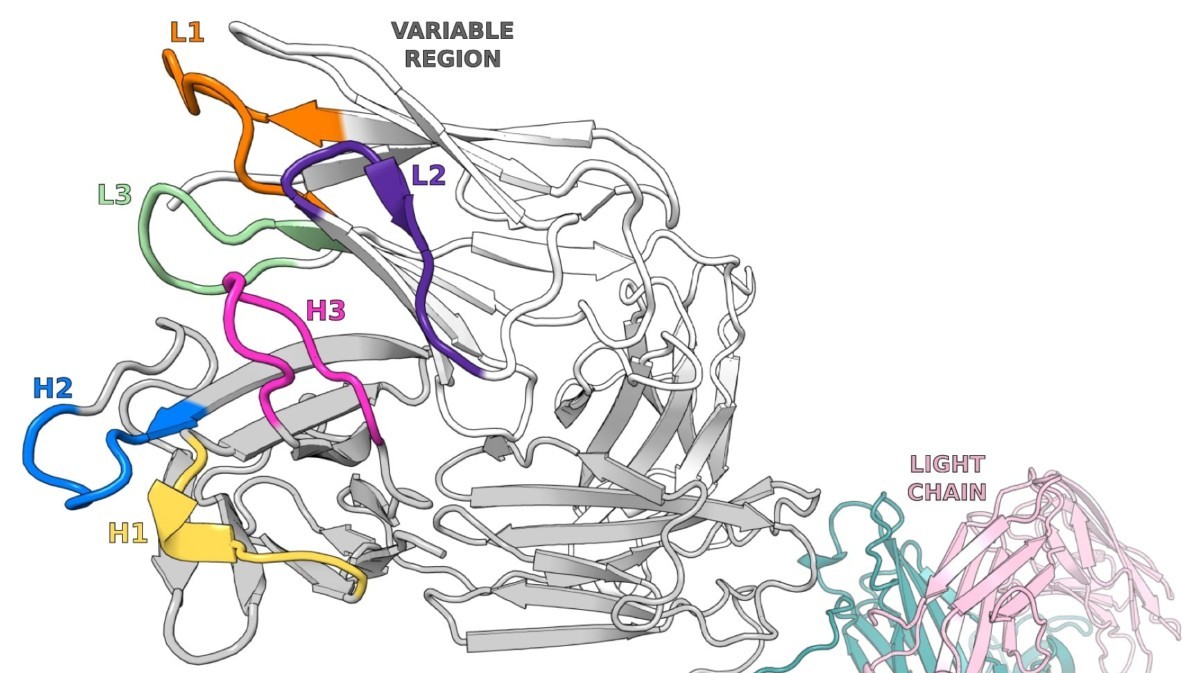
De Novo Protein Sequencing of Antibodies for Identification of Neutralizing Antibodies in Human Plasma Post SARS-CoV-2 Vaccination
To develop robust mAb biologics, it is vital to fully characterize the protein, including its primary sequence, mutations, and important post-translational modifications