Dang, H. V.; Cross, R. W.; Borisevich, V.; Bornholdt, Z. A.; West, B. R.; Chan, Y.-P.; Mire, C. E.; Da Silva, S. C.; Dimitrov, A. S.; Yan, L.; Amaya, M.; Navaratnarajah, C. K.; Zeitlin, L.; Geisbert, T. W.; Broder, C. C.; Veesler, D. Broadly Neutralizing Antibody Cocktails Targeting Nipah Virus and Hendra Virus Fusion Glycoproteins. Nat Struct Mol Biol 2021, 28 (5), 426–434. https://doi.org/10.1038/s41594-021-00584-8.
Abstract
Hendra virus (HeV) and Nipah virus (NiV) are types of Henipaviruses (HNVs) that originated in bats and can infect the human respiratory system with detrimental consequences. As enveloped, single-stranded RNA viruses, HeV and NiV use attachment (G) and fusion (F) glycoproteins on the envelope membrane to enter host cells. So far, there are no approved therapeutics or vaccines to combat the viruses in humans.
A collaborative team of researchers led by the Veesler lab at the University of Washington recently made significant progress in developing monoclonal antibody (mAb) drugs targeting F glycoproteins for NiV and HeV infection in humans. In the early stage of the research, they isolated two mAbs, 12B2 and 1F5, from hybridoma cells upon mice immunization with virus F glycoproteins. The antibodies showed potent cross-neutralizing effects against NiV and HeV infections in the biochemical and immunogenic analysis.
To develop rational strategies for antibody engineering and drug design, a detailed understanding of epitope recognition was still needed, including mAb sequences and mAb-glycoprotein structures. Thus, the researchers exploited REmAb® de novo sequencing service to efficiently resolve the primary amino acid sequences of 12B2 and 1F5. Using the sequence information, the team humanized 12B2 and 1F5 by exchanging their constant heavy and light chain domain sequences with human sequences, and generated their Fab fragments. They then successfully determined two cryo-electron microscopy structures of NiV and HeV F glycoprotein in complex with 12B2 and 1F5 Fab, respectively. The structural data revealed that 1F5 and 12B2 recognize highly conserved epitopes on the surface of the NiV F and HeV F glycoproteins.
The structural characterization of mAb-NiV/HeV binding interface inspired the Veesler lab to assess the possibility of developing antibody cocktails using the two mAbs in combination. Their preliminary tests showed positive results and are published in Nature Structural & Molecular Biology, offering strong hope for therapeutic solutions against HNV infection.
This study is a good example demonstrating the power of structure-guided drug design by utilizing state-of-the-art techniques, including cryo-electron microscopy and antibody protein sequencing. De novo protein sequencing was applied in the study to directly analyze naturally-obtained antibodies without relying on genetic information, playing an instrumental role in advancing the drug development process.
Graphical Abstract
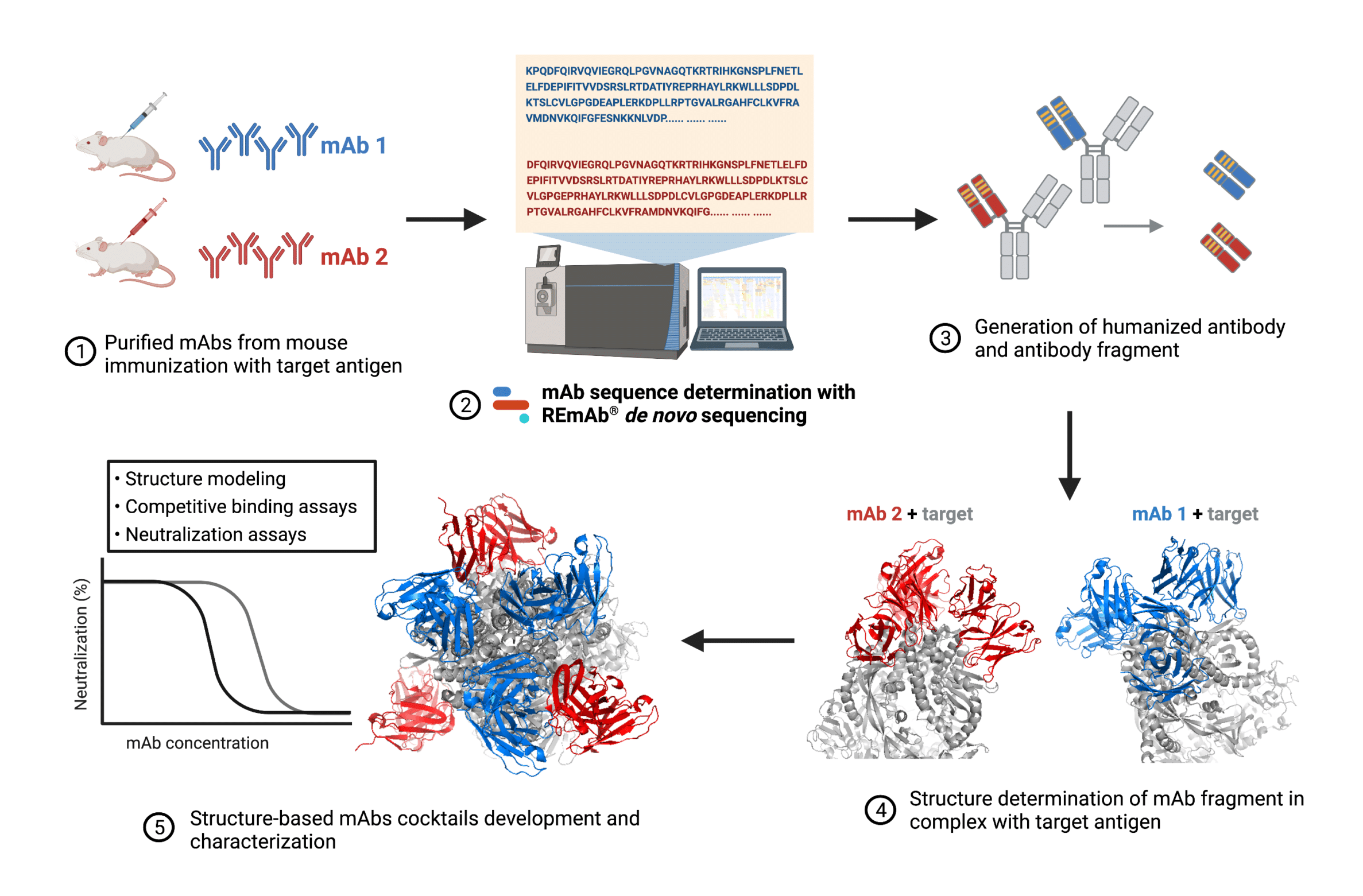
Roadmap of structure-guided design and development of monoclonal antibody cocktails against target antigen described in the study. De novo protein sequencing was exploited to derive the amino acid sequences of anti-virus mAbs. Knowledge of mAb sequence data allowed the researchers to perform antibody humanization and fragmentation. Then, by determining the cryo-electron microscopy structures of the target antigen in complex with mAb fragments, detailed epitope information was revealed, further guiding researchers to design neutralizing mAb cocktails targeting the virus.
Key Takeaways
- The Veesler lab developed a structure-based strategy for designing neutralizing monoclonal antibody cocktails against HeV and NiV viruses
- Utilizing de novo sequencing, the sequence data of anti-viral antibodies were essential for antibody engineering and structure determination to elucidate detailed epitope information
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

