Published: May 1, 2024
What Are Autoantibodies?
Autoantibodies are a type of antibody that bind to self-antigens and play a role in the balance between self-defense and self-regulation. When functioning properly, autoantibodies provide cellular maintenance, debris clearance, and homeostasis of the immune system. In addition to binding self-antigens, autoantibodies are part of the first line of defense that protect us from foreign pathogens, such as bacteria and viruses.
Dysregulation or disruption in autoantibody function leads to autoimmune disorders where the body’s own cells, tissues, and proteins are mistakenly targeted by the immune system.
How are Autoantibodies Produced?
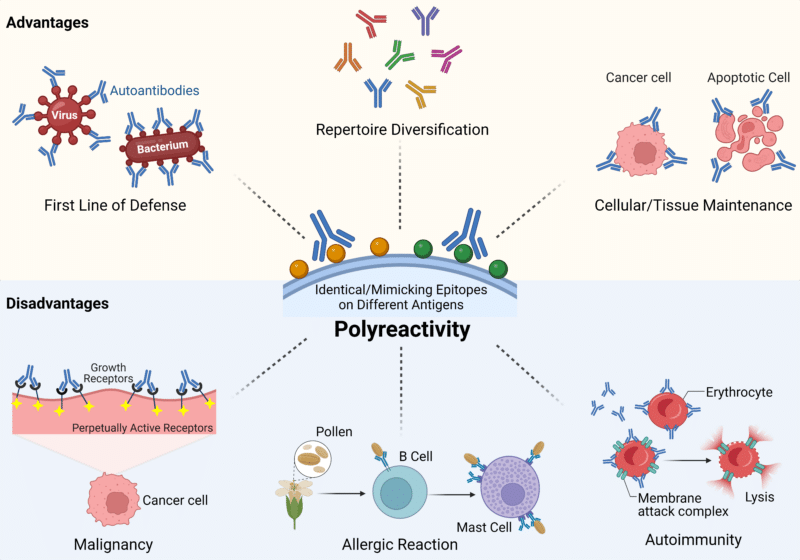
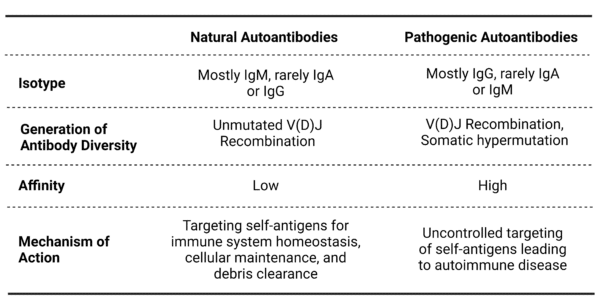
Natural autoantibodies are present in the body from birth and are typically IgM isotype antibodies. These IgM antibodies have unmutated V(D)J regions and are polyreactive with low-to-moderate binding affinity for a wide variety of antigens (Figure 1). Although they have low-to-moderate affinity, IgM antibodies have high binding avidity which allows them to bind repetitive epitopes on bacteria and viruses. This allows them to function as a surveillance system for pathogenic infections. Additionally, they provide housekeeping and homeostasis functions to the immune system due to their low-to-moderate affinity for self-antigens. For this reason, they have been termed “natural autoantibodies” as they provide important functions to the body such as immune system homeostasis, tissue maintenance and clearance of cellular debris.
Figure 1: Advantages and Disadvantages of Polyreactive Autoantibodies.
From the natural autoantibodies, somatically mutated pathogenic autoantibodies can arise due to a number of triggers or malfunctions. For example, during stressful conditions, such as chronic inflammation, self-binding IgM antibodies may trigger the immune system to undergo affinity maturation. During this process, low self-binding IgM antibodies are mistakenly selected and expanded by B cells. This leads to the production of IgG antibodies that have a high binding affinity for self-antigens. These IgG antibodies bind to and target the immune system to mistakenly attack self as if it were a foreign pathogen. This transition from natural autoantibodies to pathogenic autoantibodies is the precursor for autoimmune diseases (Table 1).
Table 1: Comparison of Natural and Pathogenic Autoantibodies
How do Autoantibodies Become Pathogenic?
Natural autoantibodies are an important part of a normal immune system and function to provide homeostasis and host defense. However, natural autoantibodies can become pathogenic and contribute to autoimmune diseases. There are several possible mechanisms in which autoantibodies can become pathogenic. Listed below are the most common reasons for autoantibodies to transition from natural to pathogenic:
Loss of Self-Tolerance Mechanisms
Self-tolerance involves a combination of mechanisms that work together to ensure that B cells producing pathogenic autoantibodies are eliminated. In the bone marrow, B cells that recognize self-antigens are eliminated by apoptosis. If the autoreactive B cells evade this mechanism, they may still be eliminated or rendered functionally inactive in secondary lymphoid organs through clonal deletion or induction of anergy. Dysregulation of these self-tolerance mechanisms can lead to production of pathogenic autoantibodies and autoimmune disease.
Molecular Mimicry and Cross Reactivity
Pathogens, such as viruses and bacteria, greatly benefit from having similar antigens to their host since it allows them to evade the immune system. However, when the host generates antibodies to fight the pathogen, this can lead to the activation of pathogenic autoantibodies. For example, S. pyogenes antigens are similar to the antigens present on cardiac valves. When the immune system produces antibodies against S. pyogenes, these antibodies can mistakenly target cardiac valves leading to rheumatic endocarditis.
Epitope Spreading
Epitope spreading refers to the diversification of epitope specificity that leads to the expansion of the immune response to include additional epitopes beyond the initial target antigen. In autoimmune diseases, tissue damage or inflammation can lead to the exposure of additional self-antigens, which may trigger the activation of autoreactive B cells and the production of pathogenic autoantibodies through epitope spreading.
Alterations in Antigen Presentation
Dysregulated antigen presentation by antigen-presenting cells (APCs) can lead to the activation of autoreactive B cells and the production of pathogenic autoantibodies. APCs may present self-antigens in a manner that promotes the activation of autoreactive immune responses, contributing to the development of autoimmune diseases.
Triggers for Pathogenic Antibodies
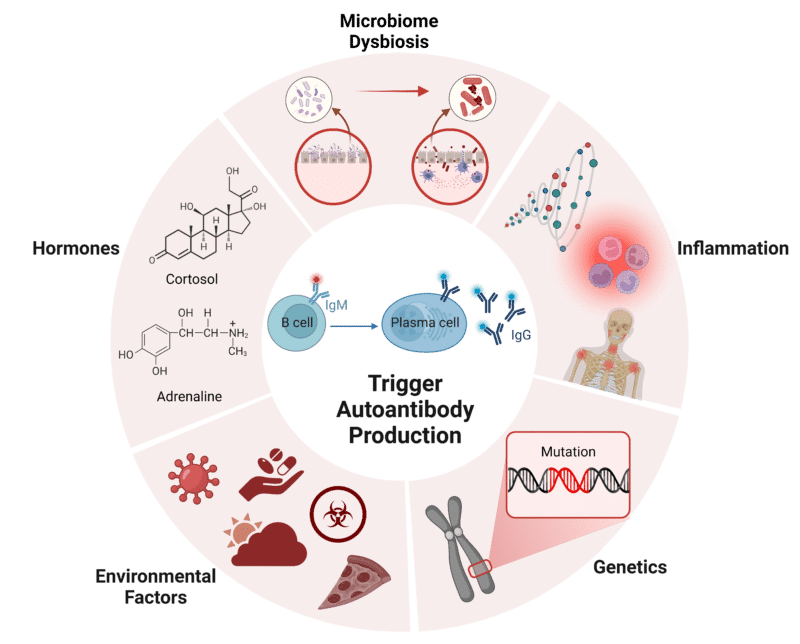
As mentioned above, dysregulation of natural autoantibodies is the driving factor of pathogenic autoantibody production. The dysregulation that produces pathogenic autoantibodies can be due to a combination of genetic, environmental, and immunological factors (Figure 2). Listed below are the key mechanisms behind pathogenic autoantibody production:
- Genetic Predisposition: Genome-wide studies have linked a number of genetic mutations to the production of pathogenic autoantibodies. These genetic mutations can affect a variety of immune system functions such as B cell tolerance and T cell activation. For example, genetic mutations affecting Fcγ receptors, such as the FCGR2A H131R polymorphism, can influence autoantibody function and immune responses.
- Environmental Factors: Many external environmental factors, such as being exposed to certain infections, dietary factors, medications, toxins, and even UV radiation have been shown to be associated with pathogenic autoantibody production. For example, Epstein-Barr virus (EBV) infections have been linked to multiple sclerosis (MS) and periodontal infections have been linked to rheumatoid arthritis. Additional examples include gluten in celiac disease and UV radiation in cutaneous lupus.
- Hormones: Hormones such as estrogen, testosterone, cortisol, and adrenaline can trigger the production of pathogenic autoantibodies. Hormonal imbalances can alter T and B cell function, cytokine production, and immune regulation that can sometimes promote pathogenic autoantibodies. This becomes evident when you consider that hormones affect the pathogenesis, clinical features, and management of systemic lupus erythematosus.
- Microbiome Disturbance: It is no secret that the composition of the human microbiome plays an important role in human health. The symbiosis between the human body and the microbiome is imperative for proper function and protection. In the case of gut microbiome dysbiosis, immune system function can be altered which can trigger pathogenic autoantibody production. For example, expansion of Prevotella copri in the gut microbiome has been associated with rheumatoid arthritis due to its homology to self-antigens.
- Inflammation: In autoimmune diseases characterized by inflammation, such as rheumatoid arthritis and systemic lupus erythematosus, self antigens released from the inflamed tissue can stimulate the production of pathogenic autoantibodies. This happens when inflammatory cytokines and chemokines that are produced during tissue inflammation also promote B cell activation and autoantibody production.
Figure 2: Triggers for Autoantibody Production.
Autoantibodies in Diagnosis and Treatment
The detection of autoantibodies is crucial for the diagnosis and management of autoimmune diseases within a timely manner, supporting positive patient outcomes. There are a variety of different tests that can be used to confirm a diagnosis, estimate disease severity, and aid in assessing prognosis. These tests include an antinuclear antibody (ANA) test, a comprehensive autoantibody panel test, an inflammatory marker test, and a flow cytometry test. For example, in systemic lupus erythematosus (SLE), antinuclear antibodies (ANAs) are used to diagnose patients with SLE. Additionally, in rheumatoid arthritis, measuring the levels of anti-cyclic citrullinated peptide (anti-CCP) in patient serum can tell you about the severity of disease manifestation and when to initiate targeted therapies.
Interestingly, many other systemic diseases, including cancer, diabetes, and neurological disorders can also be diagnosed with the use of autoantibody screening tests. Detecting autoantibodies in patient serum is a much less intrusive method for disease detection and can be used as a diagnostic tool, a prognosis indicator, and to determine treatment targets. In fact, an extensive study done by Patel et al. found that 13 autoantibodies have a very high predictive value of non-small cell lung cancer (NSCLC) patient prognosis.
In addition to diagnosis, autoantibodies can also be used for targeted therapeutics. For example, in psoriasis autoantibodies that neutralize interleukin-17 (IL-17) were found to inhibit psoriatic inflammation and reduce the severity of psoriasis in patients. In rheumatoid arthritis, autoantibodies that target tumor necrosis factor (TNF) are used to reduce inflammation and joint damage. Even harmful autoantibodies themselves can be targeted as treatment for autoimmune diseases using monoclonal antibody therapy. In addition to autoimmune diseases, autoantibodies can also be used to diagnose and treat many other diseases such as cancer. Since the development of trastuzumab, an anti-HER2 autoantibody, there has been a surge in development of autoantibody cancer therapies.
Autoantigen Discovery and Characterization
Autoimmune disease can arise from a variety of different autoantibodies targeting unique autoantigens that all lead to similar clinical presentations in patients. Autoantigens can be derived from many sources including proteins, carbohydrates, and nucleic acids. For example, joint proteins, such as type II collagen, fibrinogen, and vimentin are some of the autoantigens targeted by autoantibodies in rheumatoid arthritis. In systemic lupus erythematosus (SLE), autoantigens can be double-stranded DNA, small nuclear ribonucleoprotein, and complement system proteins. Identifying the specific autoantigen and characterizing the epitope is crucial for understanding the mechanisms of autoimmune disease and developing effective diagnostic and treatment tools.
Human autoantigen profiling technologies, such as protein arrays and display technologies, are being used to profile and characterize autoantigens and their individual epitopes. These techniques rely on hundreds to thousands of human antigens being printed on a solid surface or displayed in solution. Afterwards, human serum from a diseased patient is applied to the printed antigens and any antibody-antigen interaction can be detected. Once an interaction is detected, the autoantibody and its corresponding autoantigen can be further validated through immunoassays, such as ELISA, before being clinically validated for use as a biomarker for autoimmune disease. Importantly, techniques such as hydrogen-deuterium exchange mass spectrometry (HDX-MS) allows for the discovery of the specific autoantigen epitopes and elucidation of the mechanisms behind autoantibody pathogenicity.
Antibody Sequencing Services
De novo antibody sequencing using LC-MS facilitates comprehensive characterization of autoantibodies, aiding in both autoimmune disease research by elucidating novel targets and target validation for therapeutic interventions.
- Employ REmAb® de novo antibody sequencing to derive the amino acid sequence for in vitro diagnostic (IVD) development. Utilize validated and reproducible antibodies in IVDs for autoimmune disease diagnosis and autoantibody detection
- In vivo antibody discovery using REpAb® explores the entire circulating antibody repertoire, isolating autoantibodies associated with disease and deriving their amino acid sequences.
- HDX-MS epitope mapping identifies autoantigen epitopes, offering insights into antigenic epitopes relevant to autoimmune disease.
For more information about Rapid Novor’s services, contact our scientists!
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics