
Published: October 25, 2024
Published: October 25, 2023
Introduction
The immune system is a complex system responsible for protecting organisms from various diseases of different origins. It is composed of two types of immunity, namely innate and adaptive immunity. Innate immunity is the first line of defense and provides immediate protection, while adaptive immunity is more specific and provides long-lasting protection.
Adaptive immunity is made up of immune cells, antibodies, and memory mechanisms that work together to fight pathogens. Antibodies play a crucial role in adaptive immunity by recognizing and neutralizing pathogens. Initially, B cells produce antibodies and display them on their cell surface. However, once a B cell binds to a pathogen antigen, it stimulates the production of antibody-producing plasma B cells. This allows the immune system to have immunological memory for future infections. Although these antibodies initially have low antigen binding affinity, they acquire increased affinity over time through a process called affinity maturation.
What is Affinity Maturation?
Our immune system is constantly adapting to better protect us from harmful pathogens. One key factor in this process is called affinity maturation, which can be thought of as the immune system becoming more intelligent over time. Affinity maturation happens naturally within the body, as antibodies gain greater anti-pathogen abilities.
This process takes place in the germinal centers of secondary lymphoid organs and can last for several weeks following an infection or vaccination. Affinity maturation occurs through a series of somatic hypermutations of immunoglobulin genes in B cells, followed by selection based on their affinity strength for the antigen. The steps in the affinity maturation process are as follows (Figure 1):
- Phagocytes engulf the invading pathogen and release the pathogenic antigens into the blood and lymphatic system.
- Native B cells encounter and bind to pathogenic antigens in lymphoid organs and become activated.
- Activated B cells move to the dark zone of the germinal center (GC) where they become centroblasts.
- Centroblasts quickly multiply through a process called clonal expansion. The multiplying centroblasts all have the same B cell receptor (BCR) that binds to the same antigen.
- During clonal expansion, somatic hypermutation (SHM) introduces random genetic mutations in the immunoglobulin variable gene region at a high rate.
- Centroblasts move from the dark zone to the light zone of the GC and become centrocytes. Here, centrocytes express the mutated BCRs on their cell surface and compete for selection. This process is called germinal center selection, and it depends on the affinity of the BCR to the antigen.
- Centrocytes with the highest BCR affinity repeat the process, and with each round, the B cells have a strengthened antigen binding affinity. Centrocytes with decreased antigen affinity are not selected and undergo apoptosis.
- Once a B cell reaches optimal antigen affinity, it exits the germinal center as an antibody-producing plasma cell or a memory B cell. Plasma cells produce large amounts of antibodies to elicit a high-caliber immune response, while memory B cells protect against future infections.
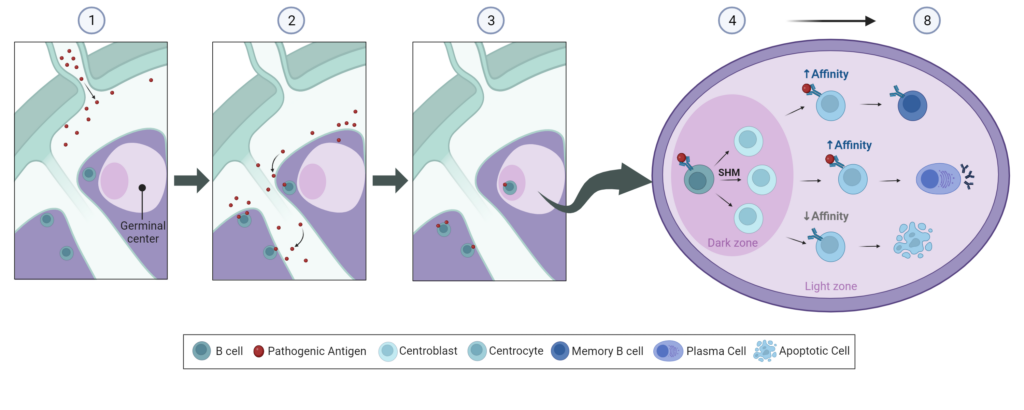
Figure 1: Overview of Affinity Maturation in the Lymph Node Germinal Center.
How Does Somatic Hypermutation Work?
B cell receptors (BCRs) are diversified through somatic hypermutation (SHM) which causes random genetic mutations in hypervariable regions (HVR) within immunoglobulin genes. These regions code for the complementarity determining region (CDR) on immunoglobulins, also known as the antigen binding site. B cells with CDR mutations that increase antigen affinity are preferentially selected, thus improving the immune system’s ability to fight against invading pathogens. This process is a major driver of adaptive immunity and occurs in the dark zone of germinal centers in secondary lymphoid tissues.
Once in the dark zone, B cells express the enzyme activation-induced cytidine deaminase (AID) which initiates the process of SHM (Figure 2). AID deaminates cytosine to uracil resulting in a uracil:guanine mismatch. Uracil-DNA glycosylase (UNG) is then recruited to remove the uracil. This repair mechanism recruits the error-prone DNA polymerase which fills in the gaps and creates mismatch mutations itself. Thousands of B cells with different mutations are generated, resulting in different affinity capabilities. B cell clones with low-affinity BCRs undergo apoptosis, while those with high-affinity BCRs bind to the antigen and repeat the whole process again.
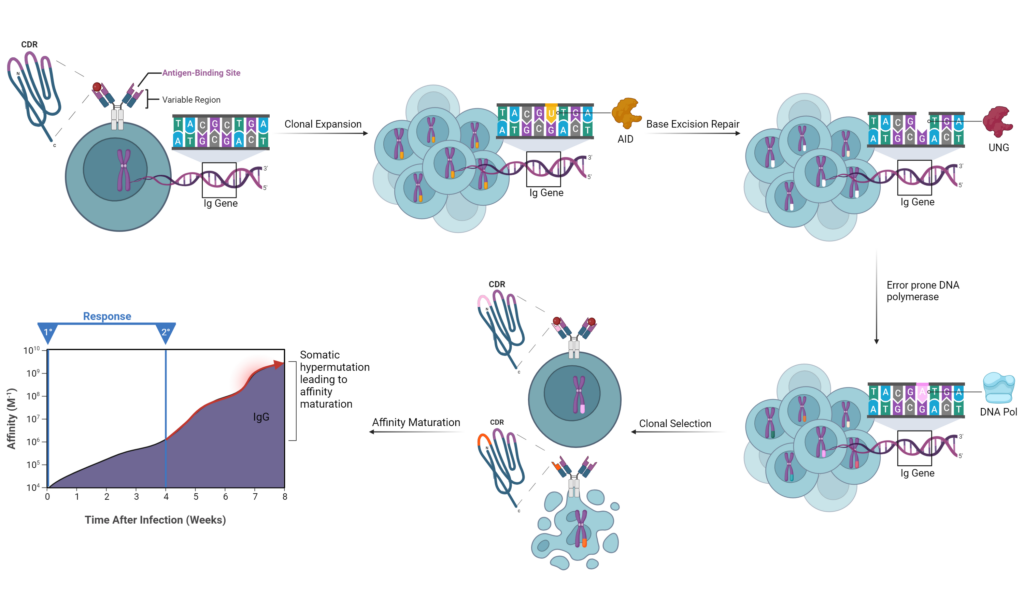
Figure 2: Overview of Somatic Hypermutation of B cell Immunoglobulin (Ig) Gene.
Applications of Affinity Maturation
Affinity maturation is a remarkable process as it utilizes natural selection to enhance the immune system’s ability to combat foreign pathogens. This is evident from the significant rise in antibody therapeutics available in the past decade. Affinity maturation holds immense significance in the fields of immunology, medicine, and biotechnology as it assists in the development of vaccines, diagnostics, and therapies.
Traditionally, scientists have achieved affinity maturation through the exploitation of the natural immune response (described above) as seen in hybridoma technology (Figure 3). More recently, scientists and engineers have developed in vitro laboratory techniques and artificial intelligence (AI) technologies to achieve affinity maturation.
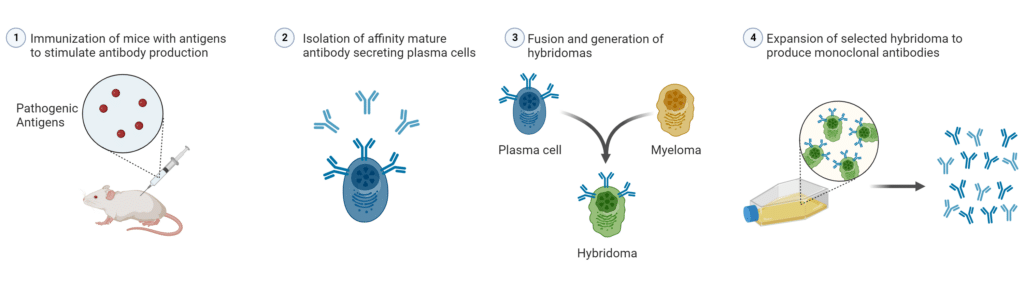
Figure 3: Exploitation of Natural Affinity Maturation through Hybridoma Technology.
In vitro affinity maturation techniques involve introducing genetic mutations in the CDR regions of immunoglobulin genes manually, followed by artificial affinity selection. Randomized unbiased mutations can be introduced using methods such as error-prone polymerase chain reaction (PCR) or DNA shuffling. Targeted mutations with a rational design can also be introduced using computation approaches. Regardless of the mutation method used, a diverse library of antibody variants is created. These antibody variants are then expressed using display technologies and subjected to stringent affinity screening techniques. Both random and targeted mutation methods lead to the generation of antibodies with increased affinity.
Scientists have used the techniques mentioned above in protein engineering to enhance the effectiveness of antibody applications. For instance, they have increased the potency of cancer immunotherapy by targeting cancer cells more efficiently. These technologies have also played a crucial role in the development of vaccines with faster response times and stronger pathogen protection. While in vitro affinity maturation techniques and AI are continuously evolving, they are not yet as highly developed as natural affinity maturation.
Polyclonal Antibody Sequencing
Understanding the antibody repertoire and the affinity maturation process is essential for comprehending immunology and its applications. In vitro and AI-based techniques for affinity maturation are unable to fully replicate the intricacies of the highly evolved in vivo affinity maturation process.
Rapid Novor’s REpAb antibody discovery using mass spectrometry leverages the natural affinity maturation process by interrogating the circulating antibody repertoire. It provides direct access to the diversity of the immune response, enabling a better understanding of immunology and its applications.
For more information on our REpAb service, contact our scientists.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

