Written by:
Written by:
Genya Gorshtein, MSc
Published: June 13, 2023
Introduction
Immunoassays have a wide range of applications in clinical diagnostics and biopharmaceuticals for quantitative, qualitative, and semi-quantitative analysis of analytes. Immunoassays such as enzyme-linked immunosorbent assays (ELISAs) and Western blots use antibodies to detect analytes by producing a measurable signal in response to antibody binding to its target antigen. Immunoassays typically require two types of antibodies: primary antibodies and secondary antibodies. Primary antibodies bind specifically to the antigen whereas secondary antibodies bind to the primary antibody. Here, we will discuss how primary and secondary antibodies work in immunoassays, how they are produced, and how to select antibodies for use in immunoassays.
How do Primary and Secondary Antibodies Work?
Immunoassays have 3 main components:
- Target analyte: the antigen detected within the sample.
- Antibody
- Primary antibody: binds to the target antigen.
- Secondary antibody: binds to the primary antibody.
- Label: a label conjugated to either the primary or the secondary antibody that produces a measurable signal.
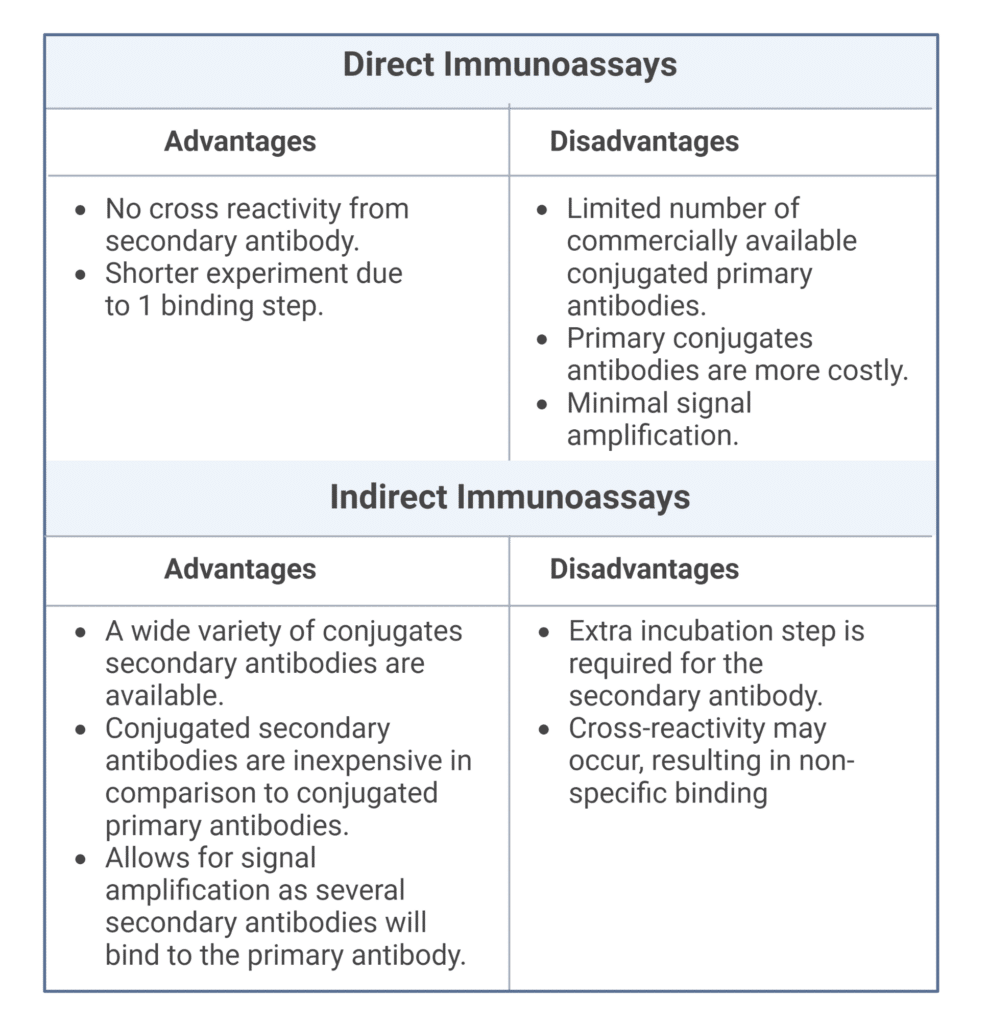
Direct vs. Indirect Immunoassays
There are two types of immunoassays, direct and indirect (Figure 1). In direct immunoassays the label is conjugated to the primary antibody and produces a measurable signal upon antigen binding (Figure 1, left). As such, direct immunoassays are a one-step detection method and do not require a secondary antibody for detection.
Indirect immunoassays utilize both primary and secondary antibodies for antigen detection (Figure 1, right). The primary antibody binds to the antigen, and the secondary antibody binds to the primary antibody and facilitates detection via the conjugated label. Secondary antibodies aid in signal amplification, as multiple secondary antibodies will bind to a single primary antibody. The amplification process allows for visualization of low-abundance antigens and improves the sensitivity of immunoassays. Direct and indirect immunoassays both have advantages and disadvantages described in Table 1.
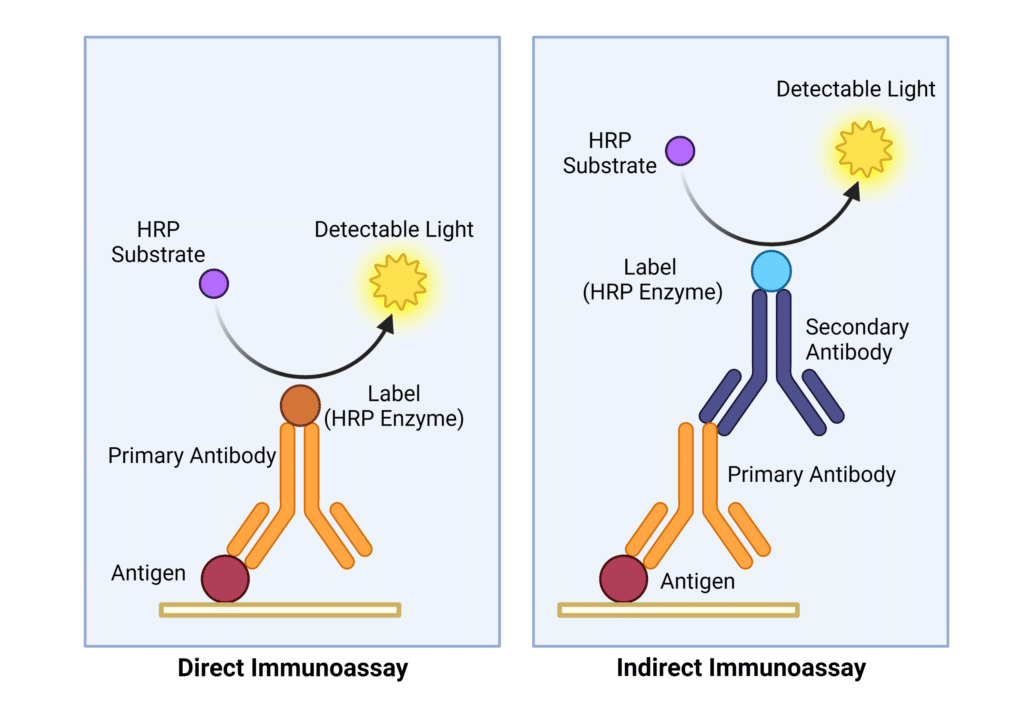
Figure 1. Direct vs. Indirect Immunoassays. Direct immunoassays utilize a primary antibody conjugated to a signal molecule. Indirect immunoassays require a primary antibody to detect the antigen, and a secondary antibody that binds to the primary antigen and produces a measurable signal. In this example, the HRP enzyme conjugated to the secondary antibody catalyzes the HRP substrate into detectable light via a chemiluminescent reaction.
Table 1. Advantages and disadvantages of direct and indirect immunoassays.
Types of Labels Used in Immunoassays
The label conjugated to the antibody produces a signal that allows for the quantitation and detection of the antigen in the sample. There are various types of labels used in immunoassays, and their selection depends on the specific requirements of the assay performed. Some common examples include:
- Enzymes: Most common type of antibody label. Enzymes commonly incorporated are horseradish peroxidase (HRP) or alkaline phosphatase (AP). Immunoassays with enzyme conjugated antibodies are analyzed through chemiluminescence or colorimetric detection methods. Enzymes are commonly used in techniques such as Western blots, immunohistochemistry (IHC) and ELISAs.
- Fluorochromes: Fluorescent dyes, such as: fluorescein isothiocyanate (FITC), Texas Red, and rhodamine. Excitation of fluorochromes at specific wavelengths produces emissions at a different and longer wavelength. Fluorochromes are widely used in techniques such as immunofluorescence microscopy.
- Radioisotopes: Antibodies labeled with radioactive isotopes are measured by the radioactivity emitted by the bound antibody complex. Radioisotopes are commonly used in radioimmunoassays (RIA), however their use has decreased due to non-radioactive alternatives.
- Biotin: Biotin is a small molecule that can be conjugated to antibodies. Biotin can be detected with streptavidin-conjugates, such as streptavidin-HRP.
How are Primary and Secondary Antibodies Generated?
Primary antibodies are generated through a combination of in vivo and in vitro technologies, such as hybridoma generation, single B cell sequencing, and polyclonal antibody sequencing. For an in-depth discussion on the technologies for antibody generation, see: Antibody Discovery Platforms.
Secondary antibodies are generated by immunizing a host animal with the antibodies from a different target animal species (Figure 2). For example, anti-rabbit secondary antibodies are raised by injecting rabbit antibodies into a goat, producing goat anti-rabbit antibodies. Common host species for secondary antibody production include goats, donkeys, and rabbits. Secondary antibodies are generated against pooled antibodies from the target species, creating a polyclonal mixture of secondary antibodies that recognize epitopes on the heavy (HC) or light chains (LC), Fab and Fc regions of the host species. Reducing cross-reactivity of secondary antibodies can be achieved by immunizing the host with a specific antibody fragment, thereby generating secondary antibodies against specific regions, such as HC- or LC-only, or Fab fragments. Antibody labels are conjugated to the secondary antibody after they are purified from the host.
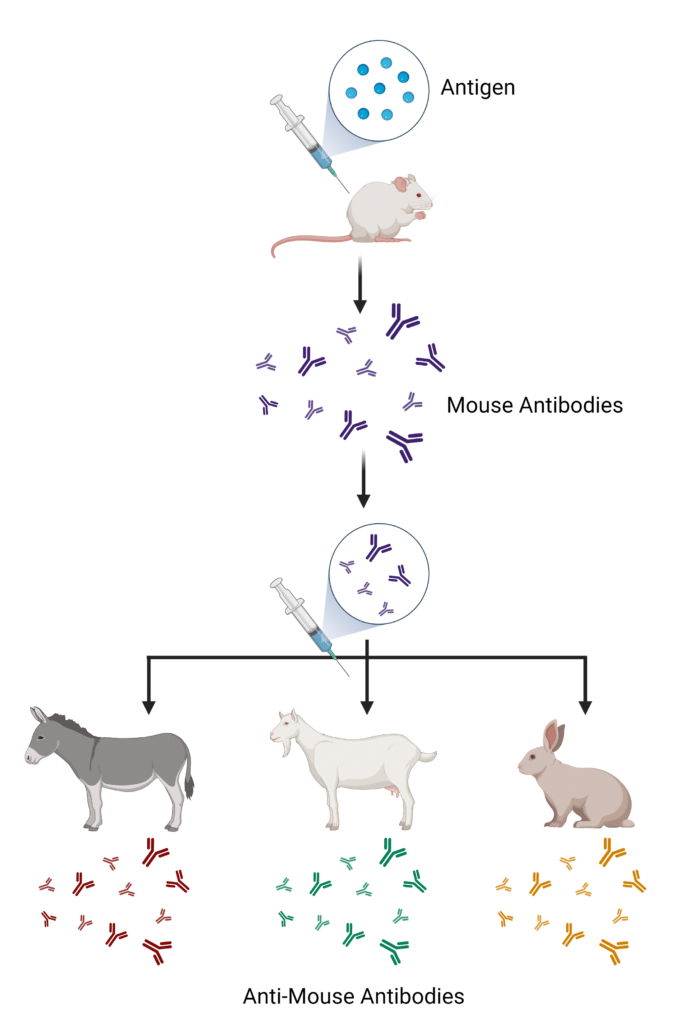
Figure 2. Process for generating secondary antibodies.
Selecting Primary and Secondary Antibodies for Immunoassays
Selecting antibodies for use in immunoassays is no easy feat. There are several considerations to be made when selecting the right antibodies, including:
- Ensure that the species of the primary antibody is different from the species of your sample to avoid cross-reactivity with endogenous immunoglobulins in the sample.
- Ensure that the primary antibody will recognize the target protein from the sample species, cross-reactivity between species is determined by sequence homology of the target epitope.
- Ensure that the primary antibody has been validated for use in the required application.
- Choose a secondary antibody raised against the host species of the primary antibody.
- Choose a secondary antibody that is conjugated to the correct label. For example, for immunofluorescence, ensure that the secondary antibody has a conjugated fluorochrome of the required wavelength.
Antibody Sequencing Service and Peptide Mapping
Immunoassays are a cost-effective and simple method for detecting and quantifying analytes with high specificity and sensitivity. However, antibody reagents are subjected to batch-to-batch variability leading to wasted time and resources and project abandonment.
Rapid Novor supports generating reliable antibody reagents for immunoassays and in vitro diagnostic development with antibody sequencing service and peptide mapping service service.
Antibody discovery services using REpAb® can be used to sequence polyclonal antibodies directly from a purified protein mixture or from immunoserum, converting a pAb to mAb with robust reproducibility.
To learn more about our services, inquire with our scientists.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics