María Gerpe, PhD
Updated: January 27, 2023
(Published: June 25, 2021)
Introduction
Antibodies or immunoglobulins (Ig) are Y-shaped glycoproteins produced by the adaptive immune system in response to antigens – substances or molecules the immune system deems as foreign. Antibodies bind to an antigen and either directly neutralize it, or activate other parts of the immune system, such as complement. Aside from single chain antibodies, and some synthetic antibodies, all antibodies have a heterotetrameric structure (two heavy chains and two light chains) that follows a quaternary order. Both the primary amino acid sequence and the 3D structure of the antibody affect its binding. In this blog, we will briefly discuss the types of antibodies that exist in nature.
Types of Antibody Structures
Specific elements of antibody structure, such as the number of Y units, and the type of heavy chain determine the isotype. Within the same species, isotypes are further classified into subtypes or subclasses. Among individuals and ethnic groups, and within the same species, there is allelic variation in subtypes; allelic variants of subtypes are known as allotypes.
In contrast, idiotypes are defined by the antigenic determinants in the variable regions of the heavy chain of antibodies and are subdivided into two groups: the antigenic determinants found in the antibody-binding regions (also known as CDRs, or paratopes), or those found in proximity to the paratope, in the framework regions (See Antibody Structure for more information).
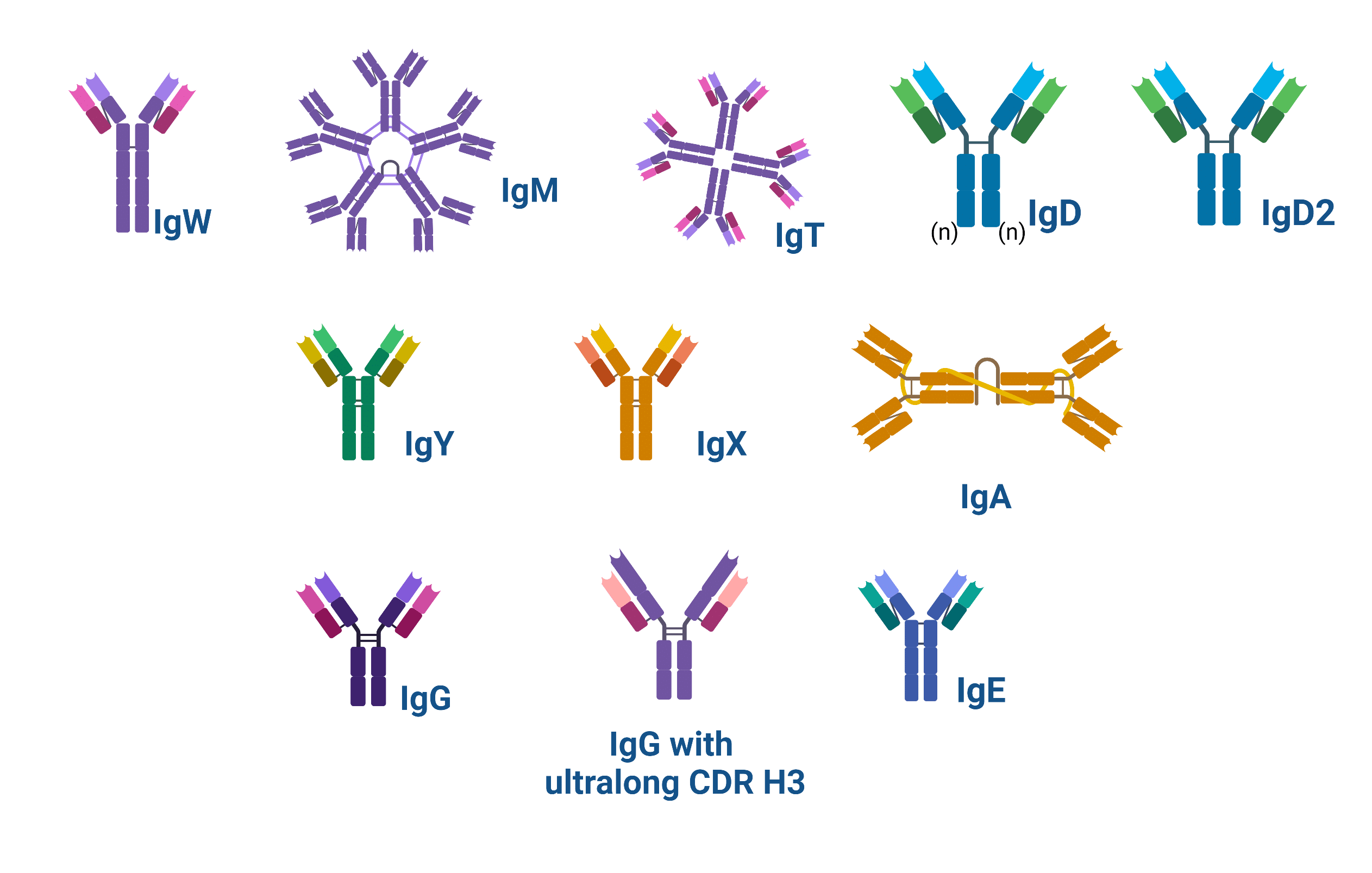
Figure 1. Schematic showing the structure of the types of antibodies found in nature
Single Chain Antibodies
There are two types of single-chain antibodies: Heavy chain antibodies (HCAb or IgVHH), expressed by camelids, and Ig new Ag receptors (IgNAR), produced by cartilaginous fish. Camelids such as camels also express heterotetrameric antibodies including their own IgG and IgA.
Tetrameric Antibodies
A total of 12 isotypes have been discovered to date across species: the immunoglobulin (Ig) isotypes A, D, E, F, G, M, P, T/Z, X, Y, and W. The first of these isotypes, IgM, has perhaps been expressed by vertebrates since the transition from sea to land; it is found in all vertebrates, except for the “living fossil”, the coelacanth, which is thought to be a primeval sea migrant.
Sharks produce IgM, and their own immunoglobulin, IgW, in addition to a single-chain antibody (IgNAR). In addition to IgM, IgD, and IgA (or its analog IgX in frogs), are present in tetrapods, except for frogs and birds (e.g. chickens), which do not express IgD, and crocodiles, which express an additional immunoglobulin (IgD2).
Amphibians also encode IgP, which is both expressed at the surface of, and secreted from cells. IgT or IgZ is found in Teleostei, the class of fish that includes zebrafish, and where both IgT and its equivalent IgZ were discovered. Some non-mammals (e.g. birds, reptiles, and amphibians) produce IgY, from which IgG and IgE are thought to evolve.
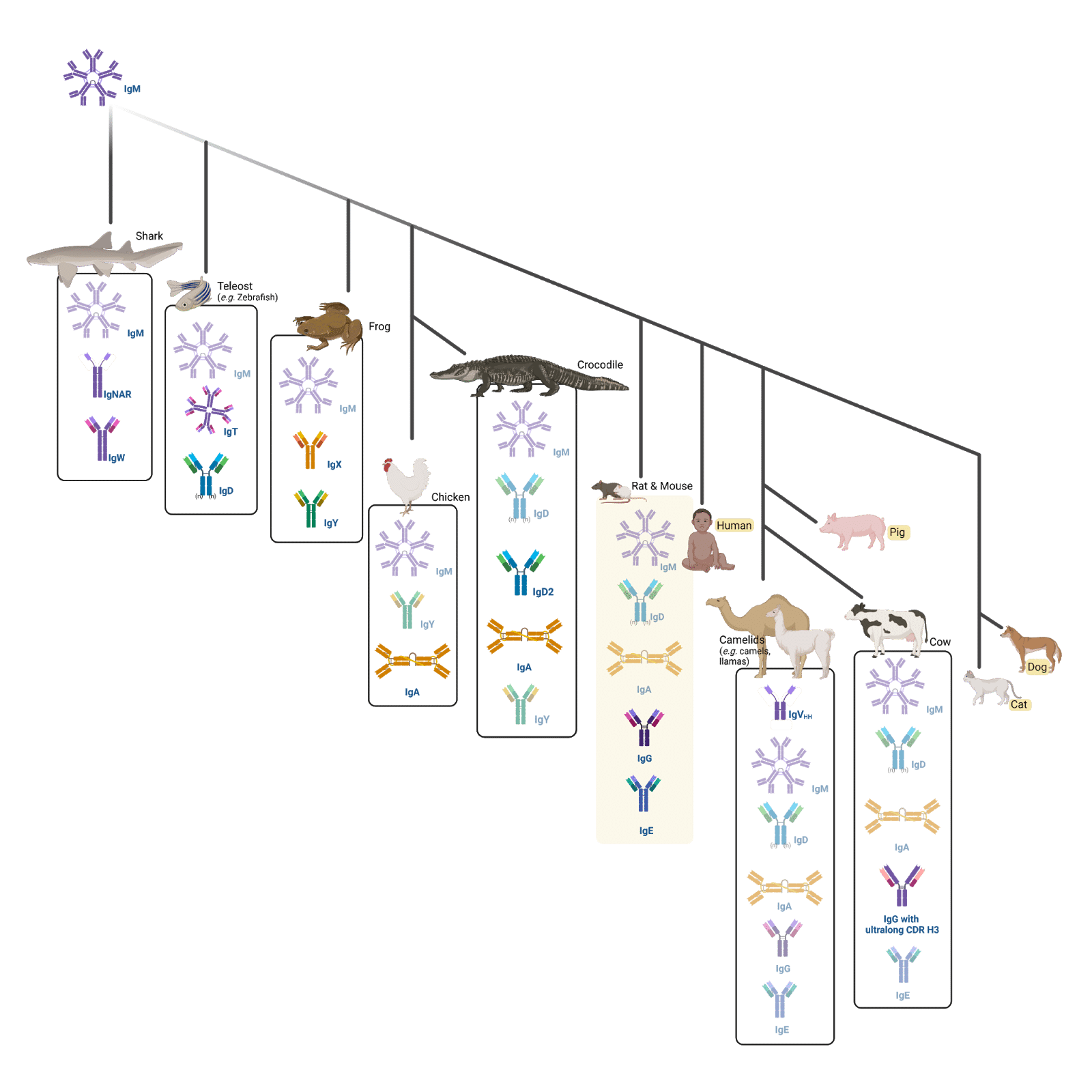
Figure 2. Infographic displaying a phylogenetic tree showing the natural evolution of immunoglobulins across different animals
In mammals, there are five main Ig types (isotypes): IgM, IgD, IgG, IgA, and IgE (highlighted in yellow in the phylogenetic tree). Humans, pigs, dogs, and cats have the same isotypes as rats and mice (the animal names are highlighted in yellow). Furthermore, cows have also evolved to express IgGs with an ultralong heavy (H) chain complementarity determining region (CDR) 3 (CDR H3). Finally, camels express IgM, IgD, IgG, IgA, IgE, and single-chain (IgVHH) antibodies.
Functions of Antibodies
IgM
The most conserved of all immunoglobulins through evolution, IgM, is the first deployed antibody in response to a foreign antigen, and particularly against pathogens during infections.
IgM can be expressed on the surface of B-cells, or secreted as either a pentamer (five Y units) or hexamer six Y units), with ten or 12 binding domains, respectively.
The binding strength (affinity) of a single IgM Y unit is lower than that of IgG’s. However, because IgM assembles into a pentamer or a hexamer, its resulting valency (the number of antigen-binding areas) or polyvalency has higher avidity (total affinity strength) than IgG’s.
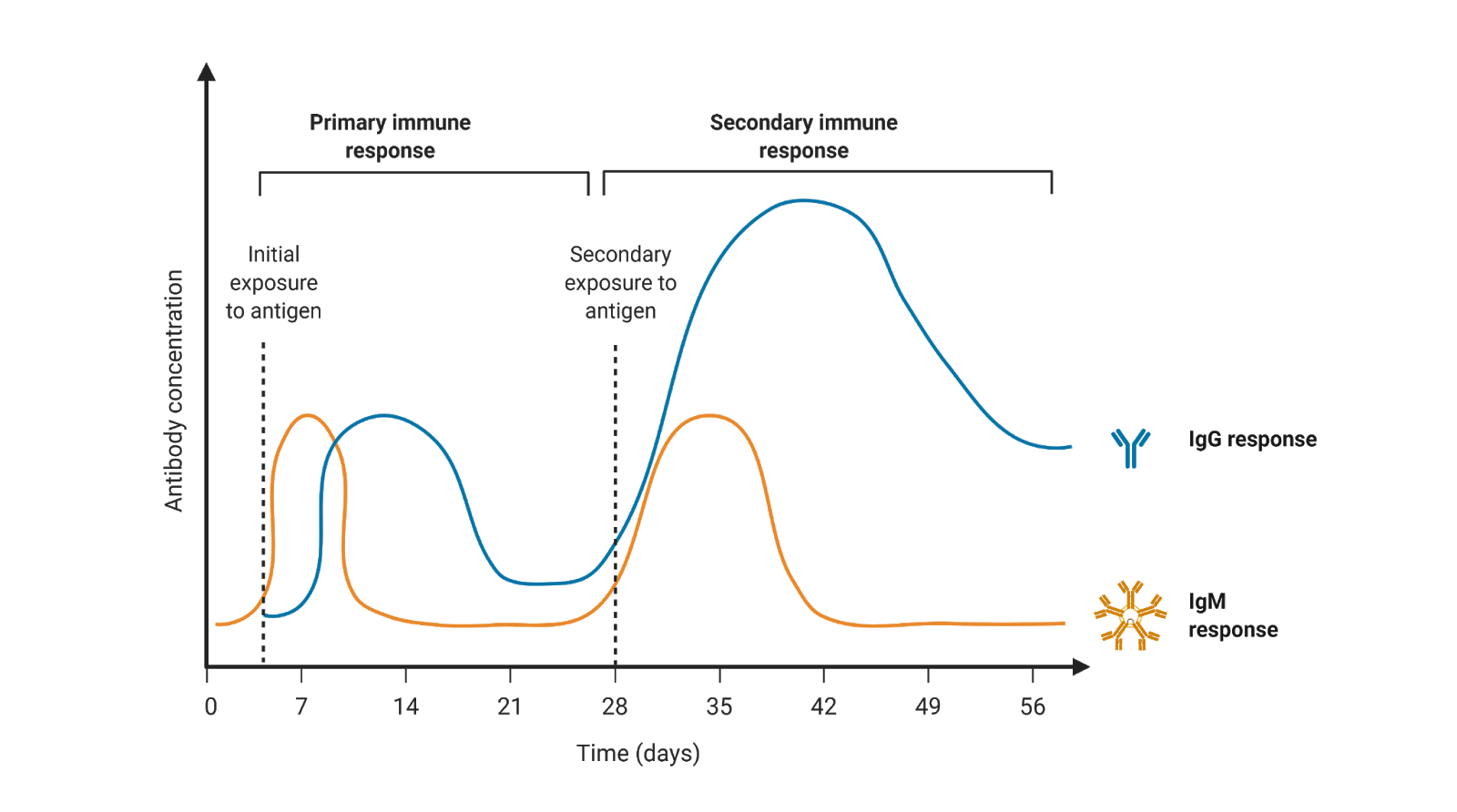
Figure 3. Concentration of immunoglobulins IgM and IgG in response to an antigen (pathogen or vaccine) across time
Because of this, IgMs can target foreign antigens found at low levels, which is typically the case for early infections. Its polyvalent nature also allows IgM to cover more “tricky” surface areas such as those of non-protein molecules like carbohydrates or lipids on the surface of pathogens like bacteria. IgMs, like IgG and IgA, have the capacity to neutralize or render a pathogen no longer infectious. Also, like IgGs, IgMs can also activate the complement system.
IgD
IgD is the second most conserved immunoglobulin, evolving shortly after IgM. IgD has very similar antigenic specificity to IgM, and it is expressed at the same time as IgM on the surface of B-cells. Though not much is known about this primordial immunoglobulin, it seems that it may play a vital role in mucosal homeostasis as an additional defense in IgA-deficient individuals,and in the regulation of the commensal microbiota in nasopharyngeal cavities of wild-type individuals.
IgA
IgA is the antibody isotype most abundantly found in the mucosal immune system. IgA, like IgM, adopts two states: it can be found as a polymer in the mucosal surface, and as a monomer in serum. In the gut, IgA is found as secretory IgA, where the polymeric IgAs consist of dimeric IgA linked by a J chain (See Antibody Structure), and a secretory component. IgA, like IgD, is critical to the regulation of the commensal microbiota and pathogenic bacteria. Because IgA is found in the gut, and is also secreted in milk, it is resistant to digestion. It also is one of the first antibodies offering passive protection from parent to infant.
IgG
IgG is the most abundantly found immunoglobulin in serum, at 10-20% of total protein in serum. Its human subtypes have moderate to excellent placenta crossing potential; notably IgG1 and specific allotypes of IgG3 have been shown to cross the placenta. As such, IgG is the first known form of passive protection from parent to infant.
IgG is secreted as a monomer, and has the highest affinity of all isotypes. It is therefore the biggest barrier to pathogens. IgG also plays an important role in complement activation.
In addition to being able to neutralize pathogens, IgGs (and IgAs) can also opsonize or tag pathogens for elimination by phagocytes. IgGs also play a role in the sensitization of many other cells of the adaptive immune system such as NK cells, mas cells
IgE
Discovered in the 1960s, IgE is the immunoglobulin associated with allergic responses. Like other immunoglobulins, it is also expressed by B-cells and plasma cells when the immune system is primed against an antigen that it deems as foreign. Interestingly, its expression is induced by the presence of two cytokines, IL-4 and IL-13. Once expressed, IgE is secreted in serum as a monomer. Compared to IgG, IgE does not have a long-lasting half-life; whereas IgG can circulate for up to 21 days, IgEs are only found in serum for a couple of days. However, during allergy responses, once bound to the affinity receptor on the cell surface of mast cells and basophils, dendritic cells, eosinophils, and platelets, it can remain bound for several weeks.
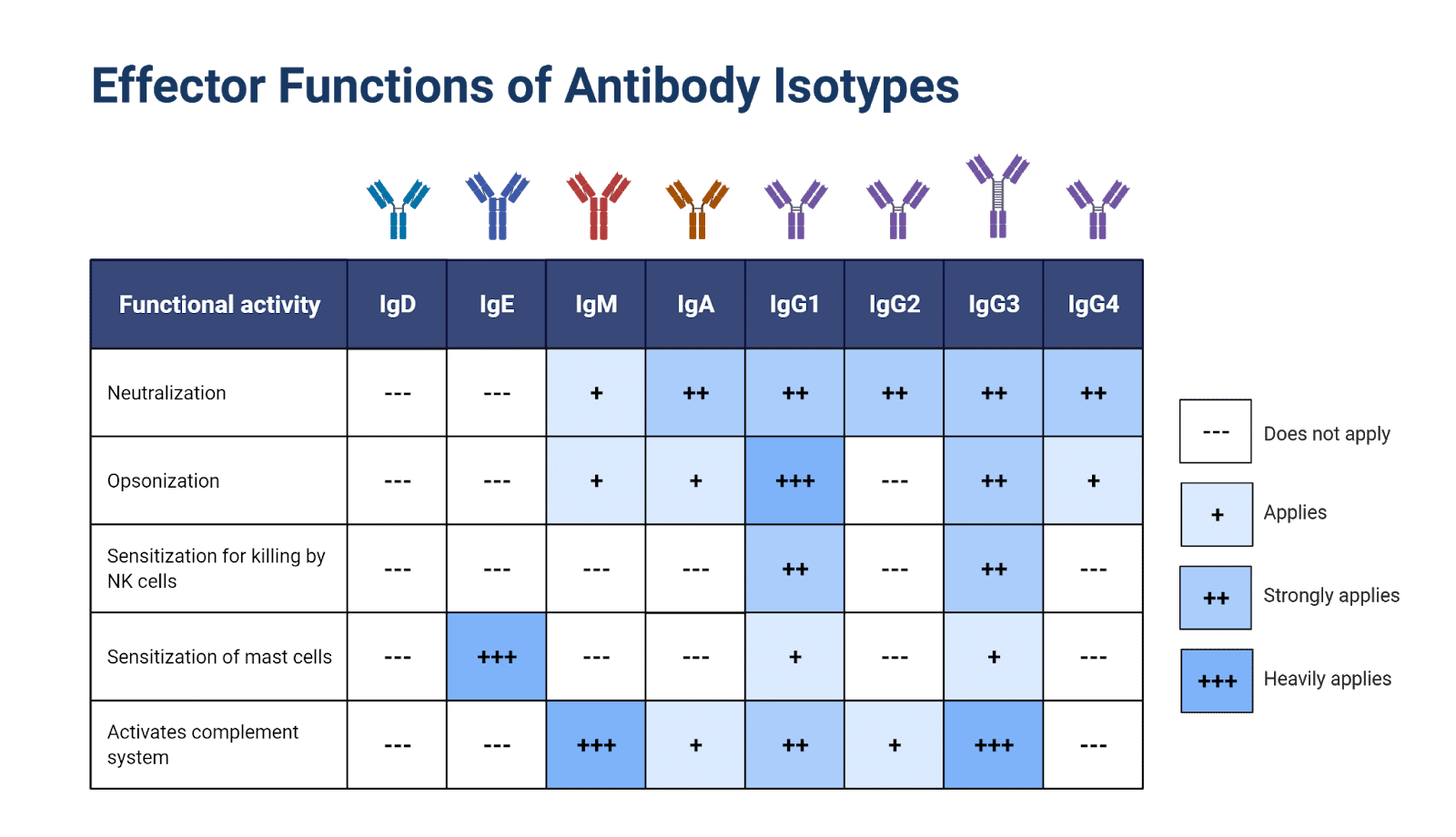
Figure 4. Effector functions of human antibody isotypes
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics