Written by: Genya Gorshtein, MSc
Written by: Genya Gorshtein, MSc
Published: June 13, 2025
Introduction
Biparatopic antibodies are a subclass of bispecific antibodies that bind two non-overlapping epitopes on the same antigen. This targeted dual engagement enhances binding avidity and can improve receptor clustering and internalization. As a result, biparatopic formats enable therapeutic applications distinct from those of conventional bispecific and monospecific antibodies.
What are Biparatopic Antibodies?
Biparatopic antibodies are engineered to bind two distinct, non-overlapping epitopes on the same antigen, which may be located on a single protein or across different regions of a multimeric complex (Figure 1).
Unlike conventional antibodies that use identical binding arms to target a single epitope, biparatopic antibodies incorporate two different antigen-binding domains (paratopes) within one molecule. While they fall under the broader category of bispecific antibodies, biparatopic antibodies differ from traditional bispecifics by targeting two sites on the same antigen rather than engaging two different antigens.
This format enables:
- Stronger antigen binding (antibody avidity) due to simultaneous engagement at two epitopes.
- Enhanced functional modulation of the target (e.g., blocking, clustering, or downregulation) due to slower disassociation rates.
- Improved specificity, especially for complex or conformational epitopes.
- Efficient internalization, a key benefit for delivering payloads in antibody-drug conjugates (ADCs).
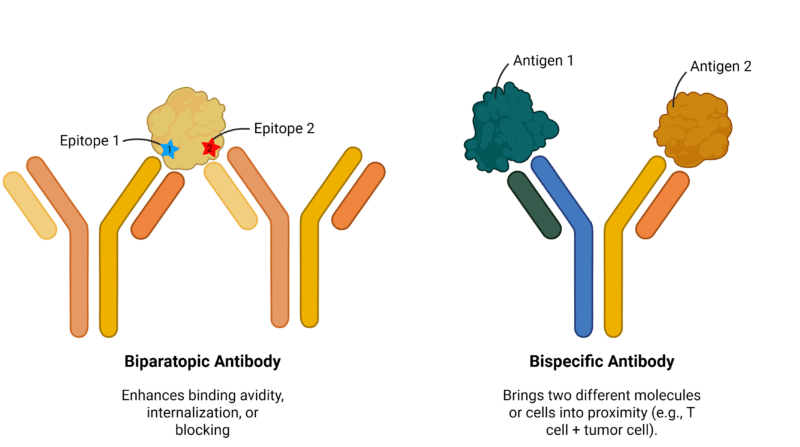
Figure 1. Comparison of biparatopic and bispecific antibodies, illustrating the key differences between biparatopic and bispecific antibody formats. Biparatopic antibodies feature two antigen-binding sites that recognize distinct epitopes on the same antigen, enhancing avidity and epitope coverage. In contrast, bispecific antibodies are engineered to bind two different antigens, enabling dual-target engagement.
How are Biparatopic Antibodies Engineered?
Designing a biparatopic antibody begins with the selection of two monoclonal antibodies that bind to distinct, non-overlapping epitopes on the same antigen. The goal is to combine their binding specificities into a single molecule to enhance avidity, improve functional activity, or overcome resistance mechanisms.
During initial antibody discovery efforts, REpAb antibody discovery enables discovery directly from the natural immune repertoire by sequencing antibodies from serum, capturing a broad and unbiased range of epitope specificities that may be missed by traditional screening or display methods. Epitope binning with SPR and epitope screening or mapping with HDX-MS can confirm the binding sites and interactions of each antibody-antigen pair.
Once two ideal monoclonal antibodies are identified and characterized, their variable regions are engineered into a single construct using formats such as:
- Tandem single-chain variable fragments (scFvs): Two scFvs connected by a flexible peptide linker to allow simultaneous binding.
- Dual-variable domain immunoglobulins (DVD-Ig): Two variable domains stacked in tandem on each heavy and light chain.
- IgG-scFv or IgG-Fab fusions: An IgG backbone fused with an additional scFv or Fab at the N- or C-terminus to create a multivalent structure.
The engineering of biparatopic antibodies often parallels that of bispecific antibodies, particularly in the use of heterodimerization strategies, such as knob-into-hole Fc designs within the CH3 domain, to ensure correct heavy chain pairing and structural integrity.
For an in-depth discussion on the diverse formats of bispecific antibodies, check out the article: The Landscape of Bispecific and Multispecific Antibodies
The choice of format depends on several factors, including desired pharmacokinetics, molecular stability, expression yield, and manufacturability. Each design must balance biological function with production feasibility to achieve a well-performing therapeutic candidate.
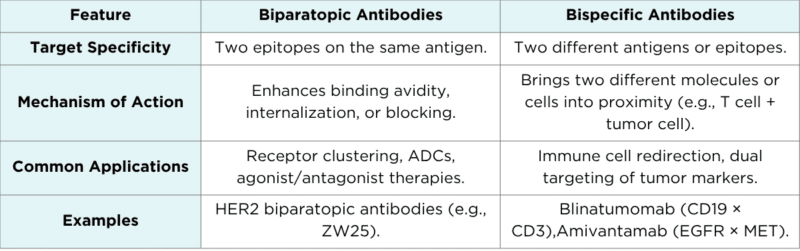
Biparatopic vs. Bispecific Antibodies: What’s the Difference?
While biparatopic antibodies are often confused with bispecific antibodies, the distinction lies in their target specificity and mechanisms of action.
Table 1. Features of biparatopic and bispecific antibodies.
These differences are crucial for therapeutic design. While bispecific antibodies are often used to bridge two separate targets (e.g., bringing a T cell into contact with a cancer cell), biparatopic antibodies work more like enhanced mono-targeting agents with fine-tuned functionality and specificity.
Engineering Biparatopic Antibodies with Rapid Novor
Like bispecific antibodies, biparatopic antibodies are notoriously difficult to produce. Their development often involves engineered sequences, peptide linkers, and post-translational modifications such as additional disulfide bonds.
- Use REpAb® antibody discovery services to discover diverse monoclonal antibody candidates directly from serum, capturing a range of epitope specificities ideal for biparatopic design.
- Use REmAb® de novo antibody sequencing services to obtain the complete amino acid sequences of candidate mAbs for constructing biparatopic antibodies.
- Guide design decisions with epitope specificity, epitope binning, and binding kinetics using HDX-MS epitope mapping and surface plasmon resonance services.
- Antibody characterization with LC-MS confirms correct sequences, chain pairing, disulfide bonds, and other modifications to ensure the antibody is produced as intended.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics