Urbano, A; Plaza, J; Picado, C; de Mora, F. Combined analytical assays for the characterization of drugs binding to human IgE: Applicability to omalizumab-bearing biosimilar candidates assessment. Biomed Pharmacother 2023, 169. https://doi.org/10.1016/j.biopha.2023.115848
Graphical Abstract
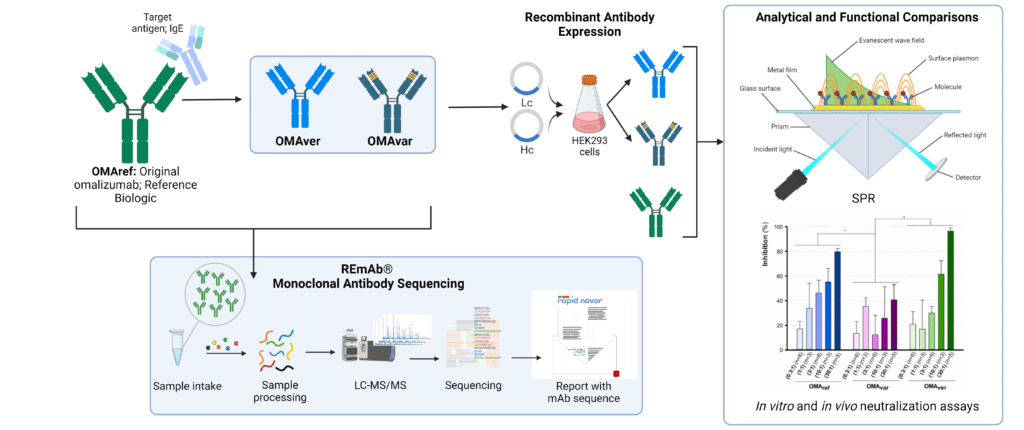
REmAb monoclonal antibody sequencing was performed to derive the complete amino acid sequences of the original reference biologic (OMAref) and two biosimilar candidates (OMAver and OMAref). Sequences were recombinantly expressed, and antibodies were subjected to analytical and functional comparative analyses.
Key Takeaways
- mAbs with identical amino acid sequences or even minor amino acid substitutions can result in slight functional differences, possibly due to differences in post-translational modifications from different expression systems and changes in 3D conformations.
- Obtaining the amino acid sequences of the reference and comparative antibodies was crucial for biosimilar development and subsequent antibody characterization.
Abstract
Biosimilars are biologic drugs that are highly similar to and have no clinically meaningful differences from an existing approved reference drug. Biologics are large, complex molecules produced using living cells, making it challenging to create identical copies. Instead, biosimilars are designed to be highly similar to the reference product in terms of structure, efficacy, safety, and quality. The development of biosimilar drugs involves sensitive and comprehensive analytical and functional comparison to minimize the risk of overlooking significant structural differences and anticipate potentially overlapping clinical features.
Researchers from the de Mora lab aimed to compare the analytical and functional properties of Omalizumab-bearing biosimilar candidates using an orthogonal analytical approach with surface plasmon resonance (SPR) and in vitro and in vivo neutralization assays.
Three anti-hIgE mAbs were studied: the original omalizumab (OMAref) produced in Chinese hamster ovary (CHO) cells, an identical sequence version produced in HEK293 cells (OMAver), and a variant with three amino acid replacements (OMAvar) produced in HEK293 cells. Amino acid sequences were confirmed using REmAb® monoclonal antibody sequencing, demonstrating 100% identity between OMAver and OMAref. Additionally, both displayed an N-linked glycosylation site on the heavy chain (HC) constant region at Asn301.
Head-to-head comparison of OMAvar and OMAver revealed that the small difference in amino acid sequence led to worsened mAb binding kinetics and hIgE recognition. While differences between OMAref and OMAver were not statistically significant, a consistent trend of higher potency for OMAver supports the hypothesis that two mAbs with an identical amino acid sequence can exhibit slight functional differences.
Understanding these distinctions between reference biologics and biosimilars is crucial, as even minor differences can lead to unintended consequences. REmAb monoclonal antibody sequencing played a vital role in providing detailed knowledge of primary amino acid sequences, aiding in biosimilar development, and facilitating downstream comparative functional assessments.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

