Abstract
In living cells, proteins work with a multitude of partner proteins and often exist in the form of multiprotein complexes. This holds true for the tuberous sclerosis protein complex (pTSC), which is an intracellular stress-sensor that integrates environmental signals, such as energy and mechanical stress, into cellular signaling pathways. The pTSC complex is a heterotrimer composed of TSC1, a 130 kDa polypeptide known as Hamartin, TSC2, a 200 kDa polypeptide known as Tuberin, and TBC1D7, a 35 kDa accessory protein that stabilizes the interaction between TSC1 and TSC2. The macromolecular composition makes the complex fragile and unstable when it’s purified in vitro, making it a challenge to obtain high resolution structural information. To date, near-complete cryo-electron microscopy (cryo-EM) density maps of pTSC were obtained by either employing chemical cross-linking or graphene oxide-coated grids during sample preparation; however, this may not reflect the true native state of pTSC.
The Mazhab-Jafari Lab from the University of Toronto recently found a way to investigate the architecture of pTSC from native sources. They developed a novel strategy to purify pTSC by using a ‘bait’ antibody to capture endogenous pTSC from animal brain tissues. The ‘bait’ antibody, which is a recombinant Fab fragment, was designed from a commercially available anti-TSC2 monoclonal antibody (mAb) that recognizes pTSC. Rapid Novor’s REmAb® de novo protein sequencing service was first used to derive the complete amino acid sequence of the variable regions of the anti-TSC2 mAb. These sequences provided the foundation for the rational design and engineering of recombinant anti-TSC2 Fab light and heavy chains, which were expressed and purified from HEK293F cells. The recombinant Fabs enabled purification of the pTSC complex directly from rat and pig brain tissue. The purified pTSC samples were visualized using negative stain electron microscopy (NSEM) and further assessed for the location of the TSC1 and TSC2 subunits using immunogold electron microscopy (IGEM). Interestingly, flexible filaments were observed in the presence of the recombinant anti-TSC2 Fabs, suggesting polymeric assembly of endogenous pTSC into filamentous super-structures.
Though recombinant protein expression and purification continues to be the standard method for generating protein samples, it is often a cumbersome process, particularly for those ‘difficult to express’ proteins and protein complexes. This study brings to light a novel path towards isolating proteins from native sources. Well-characterized antibodies can be leveraged by deriving their amino acid sequences via de novo protein sequencing and engineered with exact purpose to capture those ‘difficult to express’ proteins and protein complexes, like pTSC. The development of innovative purification strategies will only increase the accessibility to the vast array of proteins in living cells, and further streamline their structural characterization process.
Graphical Abstract
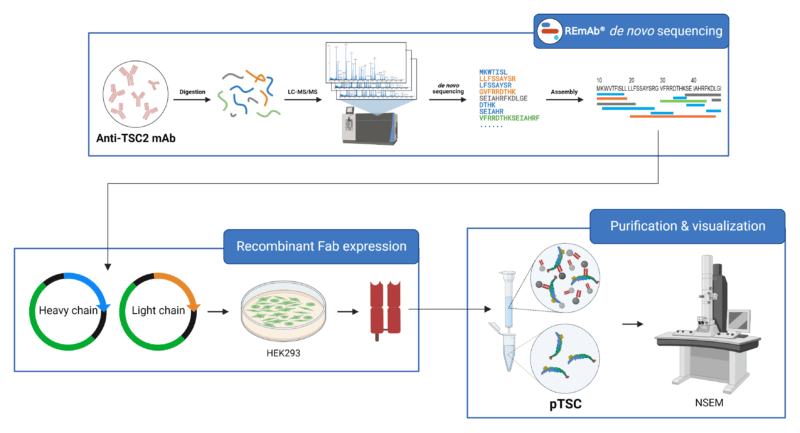
Purification strategy for pTSC using recombinant Fabs that have been designed and engineered from the commercially available anti-TSC2 mAb.
Key Takeaways
- The sequences of the variable regions of the commercially available anti-TSC2 mAb were derived using de novo protein sequencing. These sequences were leveraged in the engineering and expression of recombinant Fabs for the purification of pTSC from the native mammalian brain tissues.
- Novel approaches for recombinant protein expression and purification provide access to diverse proteins and protein complexes, especially those that may be difficult to express.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

