 Written by: Genya Gorshtein, MSc
Written by: Genya Gorshtein, MSc
Published: April 9, 2025
Introduction
Anti-idiotypic antibodies are specialized antibodies that bind to the idiotype, the unique antigen-binding region of another antibody. While these interactions can occur naturally, they are particularly valuable in the context of therapeutic drug development, where anti-idiotypic antibodies serve as essential tools for monitoring drug efficacy and immune responses.
In drug development, anti-idiotypic antibodies play a critical role in pharmacokinetic (PK) studies to measure drug levels and in anti-drug antibody (ADA) assays as positive controls.
This article will explore the different types of anti-idiotypic antibodies, their applications in drug development, and emerging technologies for their discovery and generation.
What is an Anti-Idiotypic Antibody?
An antibody idiotype is the unique set of antigenic determinants found on the variable region of an antibody, specifically within the complementarity-determining regions (CDRs) and framework regions. These idiotypes can be recognized by other antibodies and serve as targets, or epitopes, for anti-idiotypic antibodies.
Types of Anti-Idiotypic Antibodies
There are three main types of anti-idiotypic antibodies, classified based on how they interact and bind to the target antibody drug:
- Antigen-blocking anti-idiotypic antibodies: These antibodies bind directly to the paratope of the target antibody, competing with the antigen and completely blocking its interaction. They are neutralizing antibodies, and detect free, unbound drugs.
- Non-blocking anti-idiotypic antibodies: These antibodies bind to the target antibody outside of the paratope, allowing both the anti-idiotype antibody and the antigen to bind simultaneously. They detect total drugs, including free, partially bound, and fully bound forms.
- Complex-specific anti-idiotypic antibodies: These antibodies recognize the antibody drug only when it is bound to its target antigen, detecting only the drug-bound state.
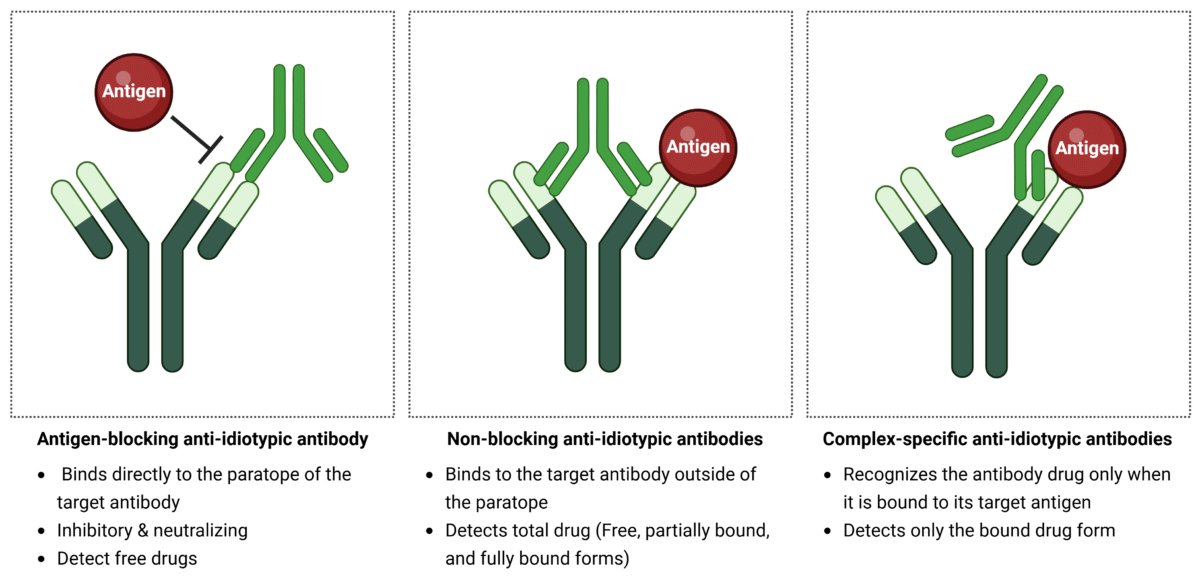
Figure 1. Types of anti-idiotypic antibody binders.
Applications of Anti-Idiotypic Antibodies in Drug Development
Anti-idiotypic antibodies are essential for developing PK assays in various formats to quantify free or total drug levels in preclinical and clinical samples. They also serve as positive controls in ADA assays.
Pharmacokinetic Assays
PK analysis studies drug concentration and metabolism to determine absorption, distribution, bioavailability, and excretion (ADBE) in patients. During preclinical and clinical trials, PK analysis provides essential guidance on dosing and dosing frequencies to optimize therapeutic efficacy and safety.
A key aspect of PK assays is the use of a high-affinity, highly specific reagent to capture the antibody drug in patient serum using ligand-binding assays such as ELISA or surface plasmon resonance (SPR). These assays measure:
- Free drug levels: The unbound drug available for target binding.
- Total drug levels: The combined bound and unbound drug in circulation.
Capture reagents can include either the drug’s target antigen or an anti-idiotypic antibody. However, anti-idiotypic antibodies are preferred due to their greater stability, lower cost, and higher reliability compared to antigens. Additionally, antigens are only suitable as capture reagents if they are soluble proteins, making them impractical for transmembrane targets. The type of anti-idiotypic antibody used determines the assay’s specificity.
Anti-Drug Antibody Assays
The development of immunogenicity assays to measure anti-drug antibodies (ADA) is a regulatory requirement by the FDA and EMEA. These assays assess the immune response to a therapeutic by detecting and quantifying ADA, which can impact drug safety and efficacy.
ADA screening and confirmation techniques include ELISA, electrochemiluminescence (ECL) immune assay, radioimmunoassay (RIA), radioimmunoprecipitation assay (RIPA), and SPR. ELISA-based assays are commonly used and are performed in two main formats:
- Direct Binding ELISA: The simplest format, where ADA from the sample is immobilized onto an ELISA plate. Enzyme-labeled secondary antibodies (e.g., HRP or alkaline phosphatase) are then introduced to detect bound ADA.
- Bridging (Sandwich) ELISA: Uses the therapeutic antibody in a two-step process. The drug is immobilized on the ELISA plate, followed by a serum wash to allow ADA binding. A second enzyme-tagged version of the therapeutic antibody is then introduced, generating a signal that confirms ADA presence.
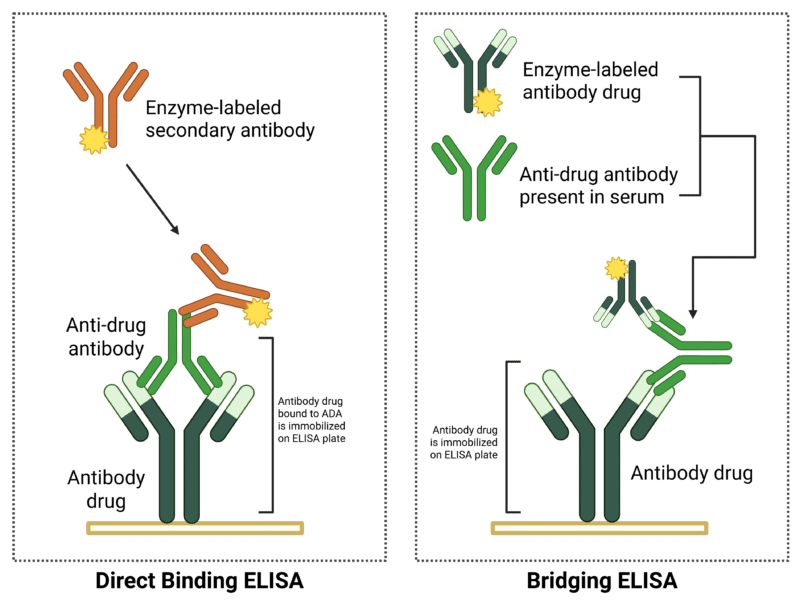
Figure 2. ELISA formats for ADA assays.
Anti-idiotypic antibodies are essential positive control reagents that ensure accuracy and consistency in assay performance. They are classified into two types: neutralizing and non-neutralizing. Neutralizing anti-idiotypic antibodies (NAbs) bind to the drug’s antigen-binding site, blocking its interaction with the target. These are used in neutralizing ADA assays to determine if patient-derived ADA inhibits drug function. In contrast, non-neutralizing anti-idiotypic antibodies do not block antigen binding but can recognize the drug or drug-ADA complexes. They are used in total ADA assays to confirm an immune response, regardless of its neutralizing effect.
According to FDA guidance on immunogenicity and ADA testing, assay sensitivity is the lowest antibody concentration that consistently yields a positive result. Immunogenicity assays must be sensitive enough to detect ADA before they impact PK, PD, safety, or efficacy. However, since positive controls are often high-affinity antibodies in the sub-nanomolar range, they may overestimate sensitivity. Thus, sensitivity should be seen as a measure of overall assay performance rather than a strict detection threshold for ADA in patients.
As PK and ADA assay reagents, monoclonal antibodies (mAbs) are preferred due to their consistency and reliability. However, polyclonal antibodies (pAbs) can be a cost-effective and faster-to-generate alternative. Despite this, mAbs are generally favored as neutralizing controls in ADA assays, as polyclonal antisera may contain antibodies targeting multiple epitopes, potentially reducing assay consistency.
Discovery and Generation of Anti-Idiotypic Antibodies
The FDA recognizes several methods for generating positive controls and reagents for ADA and PK assays, including animal immunization, hybridoma generation, B-cell sequencing, and phage display.
However, since anti-idiotypic antibodies must specifically bind to the antigen-binding region, these methods often lack functional validation before screening.
A protein-first approach using mass spectrometry-based polyclonal sequencing improves discovery efficiency by selectively isolating functional anti-idiotypes through an enrichment workflow before sequencing.
Function-Driven Discovery of Anti-Idiotypic Antibodies
To develop anti-idiotypic antibodies, an alpaca was immunized with an IgG, and serum was collected for IgG purification using protein A/G.
A function-first approach was applied, utilizing specialized enrichment strategies to select functional antibodies:
- Negative depletion was performed using a similar antibody antigen, with the same constant region but a different variable region to remove non-specific antibodies.
- Positive enrichment followed, employing affinity-based purification of the antigen’s F(ab’)2 fragment to selectively isolate idiotypic binders.
After enriching for idiotype binders, the purified pAbs underwent de novo sequencing using mass spectrometry, advanced biochemical and protein separation techniques, and machine learning. This process enabled the reconstruction of full-length, paired heavy and light chain sequences, which were recombinantly expressed and evaluated for idiotypic binding.
In this alpaca case study, out of 18 recombinantly expressed sequences derived from de novo sequencing, 16 bound specifically to the variable region of the antibody antigen and not to the constant regions.
By isolating functional anti-idiotypes before sequencing, REpAb antibody discovery enabled precise functional selection through carefully designed enrichment strategies. This approach enhances efficiency compared to traditional methods, identifying a diverse pool of candidates with defined functional properties.
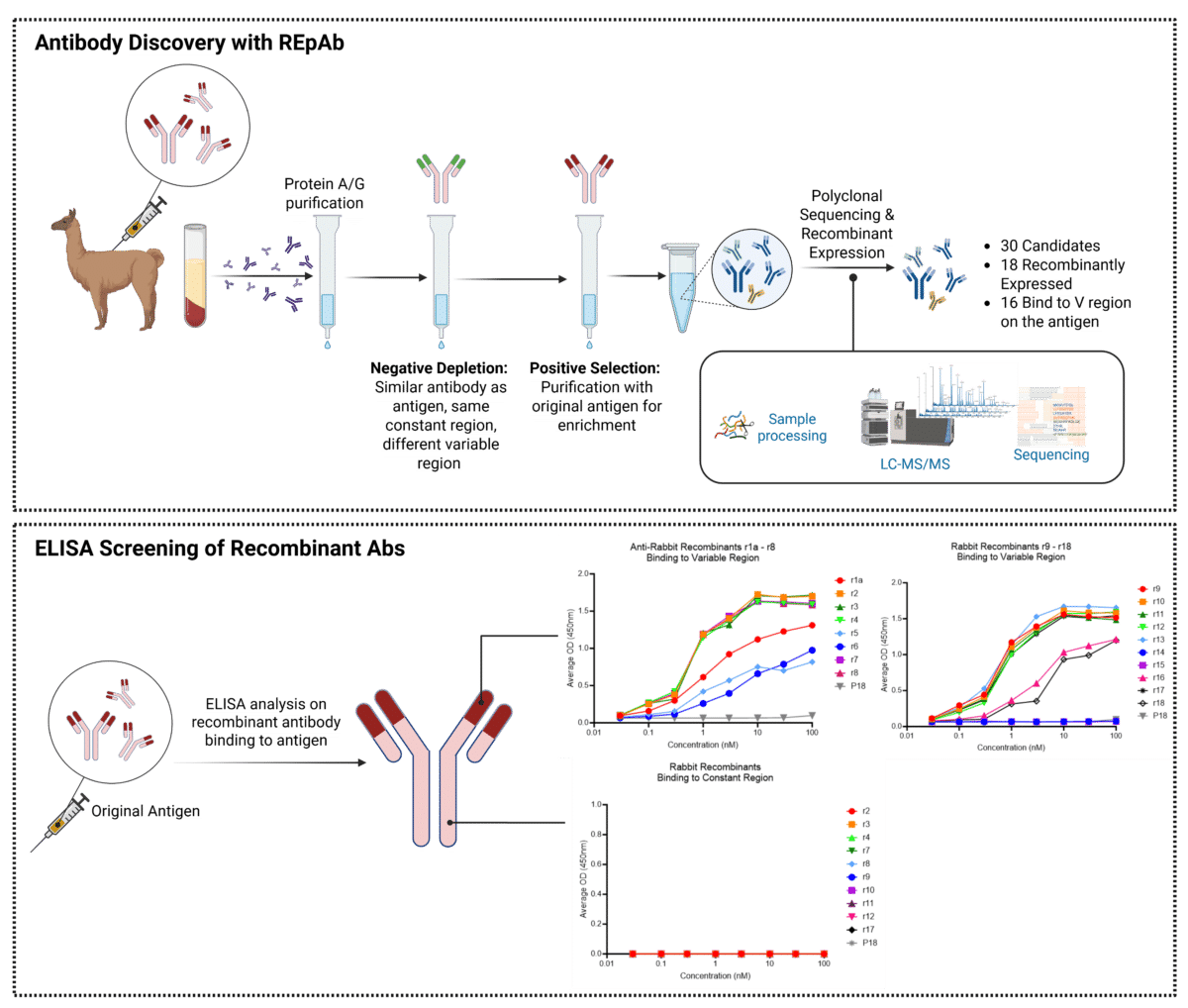
Figure 3. Alpaca case study for the discovery of anti-idiotypic antibodies using REpAb polyclonal antibody sequencing. Top: An alpaca was immunized with an IgG antigen, and immunoserum was collected, processed through negative depletion and positive enrichment to isolate functional idiotypic antibodies, then sequenced de novo using mass spectrometry. Bottom: 18 recombinant antibodies were expressed and tested via ELISA against the variable and constant regions of the original IgG antigen, confirming specific anti-idiotypic binders and the successful depletion of Fc and constant region binders from the sequencing pool.
Anti-Idiotype Antibody Discovery with Rapid Novor
Rapid Novor’s antibody discovery platform, powered by de novo polyclonal sequencing, delivers developable, functional mAbs by starting with functional antibody proteins.
For more information on how REpAb antibody discovery works, check out our Nature Communications paper on sequencing functional antibodies from human serum.
Contact our scientists and learn more about how REpAb antibody discovery service can improve your antibody discovery efforts.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

