Wang, J.; Lundström, S. L.; Seelow, S.; Rodin, S.; Meng, Z.; Astorga-Wells, J.; Jia, Q.; Zubarev, R. A. First Immunoassay for Measuring Isoaspartate in Human Serum Albumin. Molecules 2021, 26 (21), 6709. https://doi.org/10.3390/molecules26216709.
Abstract
The ongoing pandemic has reinforced the need for in vitro diagnostics to globally surveille emerging pathogens and provide better medical care. In particular, immunoassays are favoured due to their affordability, ease, and speed. Nevertheless, the combination of rapidly evolving pathogens, and more complex diseases resulting from increasing life expectancy worldwide require more sensitive and specific immunoassays in the nick of time. To increase sensitivity, immunoassay development can benefit from exploiting industry-leading technologies such as de novo protein sequencing.
Recently, the Zubarev lab at the Karolinska Institutet, Sweden, successfully exploited Rapid Novor’s REmAb® protein sequencing service to develop a novel, high sensitivity, quality and throughput immunoassay for Alzheimer’s disease (AD). In the study, isoaspartate (isoAsp) accumulation in human serum albumin was used as a biomarker for the detection of AD. A set of isoAsp-specific monoclonal antibodies were first generated in hybridoma cells. To further characterize the antibodies, the Zubarev lab resorted to de novo sequencing, which helped them quickly obtain 100% sequence coverage and confirm ambiguous amino acids. Based on the selected antibody, an indirect enzyme-linked immunosorbent assay (ELISA) against isoAsp in human serum albumin was developed that could be used as an auxiliary tool in AD diagnostics.
This is the first report of a monoclonal antibody (mAb) specific to IsoAsp in human serum albumin and the first immunoassay to measure a specific Alzheimer’s biomarker in blood. The study showcased the power of proteomics in developing an in vitro diagnostic of high quality and throughput in a short time frame.
Graphical Abstract
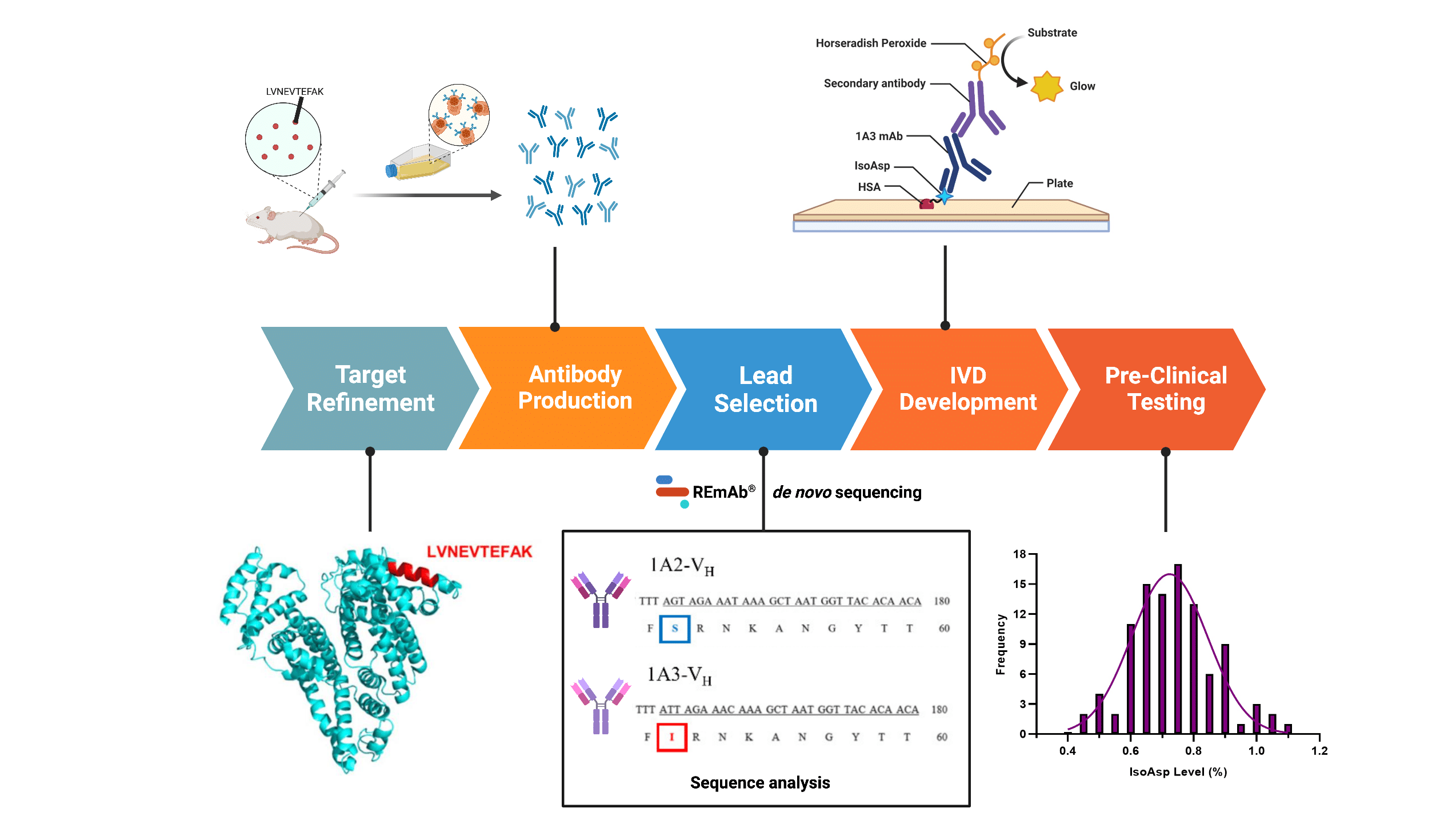
Schematic illustrating the stages involved in the in vitro diagnostics (immunoassay) development (coloured boxes) and techniques utilized in each stage by the published study. REmAb® de novo protein sequencing was exploited in lead selection for sequence analysis of selected monoclonal antibodies. These processes allowed researchers to quickly identify a novel antibody against isoAsp in human serum albumin, facilitating the development of an ELISA test for AD diagnostics.
Key Takeaways
- Isoaspartate in human serum albumin can be used as a biomarker to develop in vitro diagnostic tools for Alzheimer’s disease
- A novel immunoassay leveraging antibody de novo sequencing was recently developed based on anti-isoAsp antibodies
- Knowledge of the antibody sequence was instrumental in the lead selection and advancement of the immunoassay development
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

