Carmen Martin-Alonso et al. Priming agents transiently reduce the clearance of cell-free DNA to improve liquid biopsies. Science 383, (2024). DOI:10.1126/science.adf2341
Key Takeaways
- ctDNA shows promise for non-invasive cancer detection and monitoring through standard blood tests, but its effectiveness is limited by degradation and clearance mechanisms.
- A novel approach using DNA-binding monoclonal antibodies with blocked FcγR-binding improves cfDNA recovery by delaying ctDNA clearance.
- DNA-binding mAbs boosted ctDNA recovery in tumor-bearing mice by more than 10-fold, enhancing liquid biopsy sensitivity and ctDNA detection capabilities.
Summary
Circulating tumor DNA (ctDNA), released into the bloodstream by tumor cells, is a promising biomarker for cancer detection, providing a non-invasive means for diagnosis and monitoring of disease via standard blood tests. However, the utility of cell-free DNA (cfDNA) is limited by its scarcity, attributed to degradation by circulating nucleases and organ-mediated clearance. In oncology, ctDNA-based screening tests exhibit low sensitivity (~20 to 40% for Stage I cancer) and can yield inconclusive results in up to 40% of patients with advanced cancer. To enhance the sensitivity of liquid biopsies for ctDNA detection, mitigating ctDNA clearance from the bloodstream presents a viable strategy.
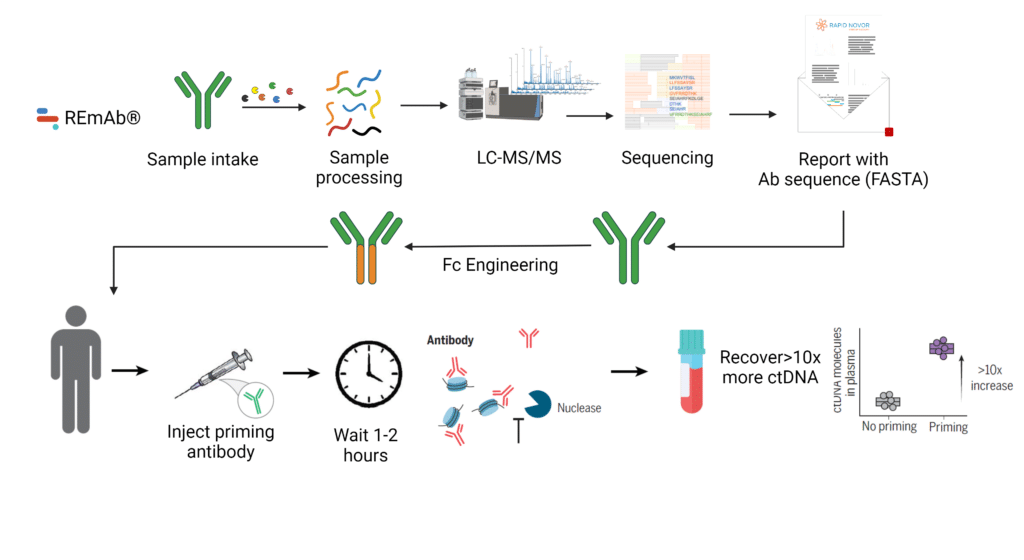
Researchers from the Tabrizi and Adalsteinsson labs at the Koch Institute For Integrative Cancer Research and the Broad Institute of MIT and Harvard aimed to develop a universal strategy to improve cfDNA recovery from blood samples. Their method involved the utilization of intravenous priming agents to momentarily stall ctDNA clearance. They identified DNA-binding monoclonal antibodies (mAbs) capable of binding to both free and histone-bound DNA, thereby protecting the DNA from nucleases. However, they encountered challenges as FcγR-mediated clearance of dsDNA bound to mAbs offset the benefits of extended dsDNA stabilization.
To address issues associated with FcγR-mediated clearance, they elucidated the complete amino acid sequence using Rapid Novor’s REmAb® de novo monoclonal antibody sequencing service. This enabled Fc engineering of the DNA-binding mAb, abrogating FcγR binding and enhanced ctDNA recovery.
Administering the engineered DNA-binding mAb at various doses 2 hours before blood collection led to a significant increase in ctDNA recovery by more than 10-fold in tumor-bearing mice. This breakthrough effectively addresses the challenge of low ctDNA quantities, which frequently limits the sensitivity of liquid biopsies for oncological purposes. Moreover, it enables non-invasive cancer diagnosis and monitoring, potentially improving patient care and treatment outcomes.
Graphical Abstract
REmAb® de novo monoclonal antibody sequencing service was employed to derive the complete amino acid sequence of the DNA-binding antibody. This information facilitated Fc engineering to obstruct FcγR-binding, making it suitable as a DNA-priming agent for the development of in vitro diagnostics for ctDNA detection in oncology applications.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics