Kanack A, Leung N, and Padmanabhan A. Diagnostic Complexity in Monoclonal Gammopathy of Thrombotic Significance. NEJM. 2024 Nov20;391(2). doi/abs/10.1056/NEJMc2409428
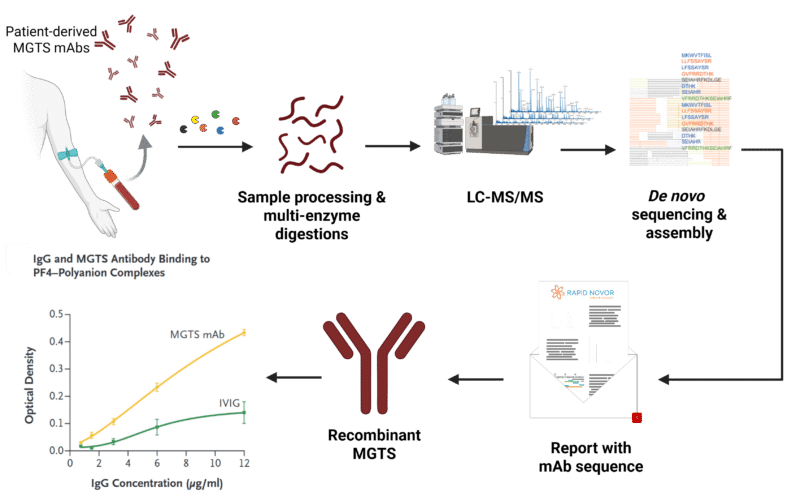
Patient-derived MGTS antibodies were isolated and de novo sequenced using the REmAb antibody sequencing service. The resulting MGTS monoclonal antibody was recombinantly expressed and validated through in vitro assays, including ELISAs comparing its binding to PF4–polyanion complexes with that of pooled human IgG (IVIG), to confirm MGTS diagnosis.
Key Takeaways
- Researchers isolated a monoclonal antibody from a patient with suspected MGTS and successfully sequenced it using REmAb® de novo antibody sequencing, without prior genetic information.
- De novo antibody sequencing enabled the development of a diagnostic assay, confirming MGTS and demonstrating its value in medical applications.
Summary
Monoclonal gammopathy of thrombotic significance (MGTS) is a prothrombotic condition involving platelet-activating monoclonal antibodies against platelet factor 4 (PF4), typically diagnosed using assays like PF4–polyanion ELISA and serotonin-release assay (SRA).
At Mayo Clinic, researchers Dr. Adam Kanack, Dr. Nelson Leung, and Dr. Anand Padmanabhan, faced challenges when a patient with MGTS symptoms tested negative in standard diagnostic assays. Further investigation revealed that the patient’s blood sample activated platelets only in the presence of PF4, suggesting the presence of prothrombotic (clot-inducing) antibodies.
To confirm MGTS, the patient’s monoclonal antibody was isolated and sequenced using REmAb® de novo antibody sequencing services from Rapid Novor, followed by recombinant expression. The recombinant antibody demonstrated PF4–polyanion binding and platelet activation, mirroring patient serum results and confirming the diagnosis of MGTS.
De novo antibody sequencing proved critical in developing a diagnostic assay for this difficult-to-detect condition, ultimately improving patient care.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

