Marhelava, K., Fidyt, K., Pepek, M. et al. LILRB1-directed CAR-T cells for the treatment of hematological malignancies. Leukemia 39, 1395–1411 (2025). https://doi.org/10.1038/s41375-025-02580-z
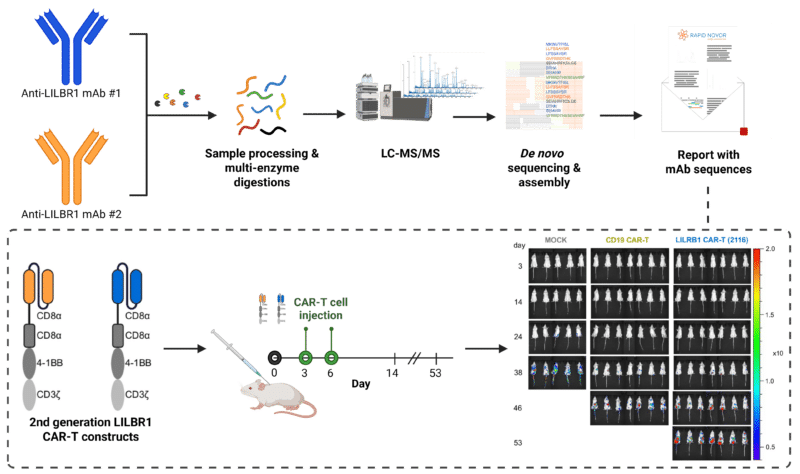
De novo sequencing of two anti-LILRB1 monoclonal antibodies using LC-MS/MS, which were used to design scFvs for second-generation CAR constructs. Engineered LILRB1-targeting CAR-T cells were injected into mice bearing B-ALL tumors. Bioluminescent imaging revealed that LILRB1 CAR-T treatment significantly reduced tumor burden compared to control and CD19 CAR-T cells.
Key Takeaways
- New targets like LILRB1 present promising alternatives to traditional CAR-T antigens by allowing for more selective and tumor-specific targeting.
- De novo antibody sequencing supports precise antibody engineering and scFv design, streamlining CAR development against previously unaddressed antigens.
Summary
Ideal antigens for CAR-T targets should be uniformly expressed on malignant cells but absent from normal tissues to minimize toxicity. Even well-established targets like CD19 and B-cell maturation antigen (BCMA) fall short of this gold standard.
Researchers in the laboratories of Dr. Winiarska and Dr. Firczuk at the Medical University of Warsaw applied cell surface proteomics on B-cell acute lymphoblastic leukemia (B-ALL) samples to identify LILRB1 (Leukocyte immunoglobulin-like receptor subfamily B member 1) as a novel CAR-T target.
To develop CAR-T cells targeting this antigen, they selected two LILRB1-specific monoclonal antibodies (mAbs). Using REmAb® de novo monoclonal antibody sequencing service, the anti-LILBR1 mAbs were sequenced by LC-MS/MS, and the variable domains were then used to design single-chain variable fragments (scFvs). These scFvs were incorporated into a second-generation CAR construct.
The LILRB1-targeted CAR-T cells specifically killed LILRB1-expressing tumor cells both in vitro and in vivo, while sparing LILRB1-negative cells. Future work will focus on refining the scFv and testing alternative CAR backbones to enhance therapeutic efficacy.
Antibody sequencing accelerates engineering novel therapeutics against new targets, speeding up target validation and preclinical studies. This enables faster design, testing, and optimization of promising therapies.
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics

