Written by: Jenna Kerry, MSc
Written by: Jenna Kerry, MSc
Published: May 7, 2025
Introduction
If the immune system were an orchestra, cytokines would be the conductors. Cytokines are small signaling proteins that play a key role in regulating the immune system’s response to infections, injuries, and diseases. However, like any finely tuned system, balance is key. Too little cytokine activity can impair the immune response, while too much can lead to chronic inflammation and autoimmune disorders. Understanding cytokines is essential for advancing therapeutics for a wide range of conditions, from infections and cancer to autoimmune diseases and inflammatory disorders.
What are Cytokines?
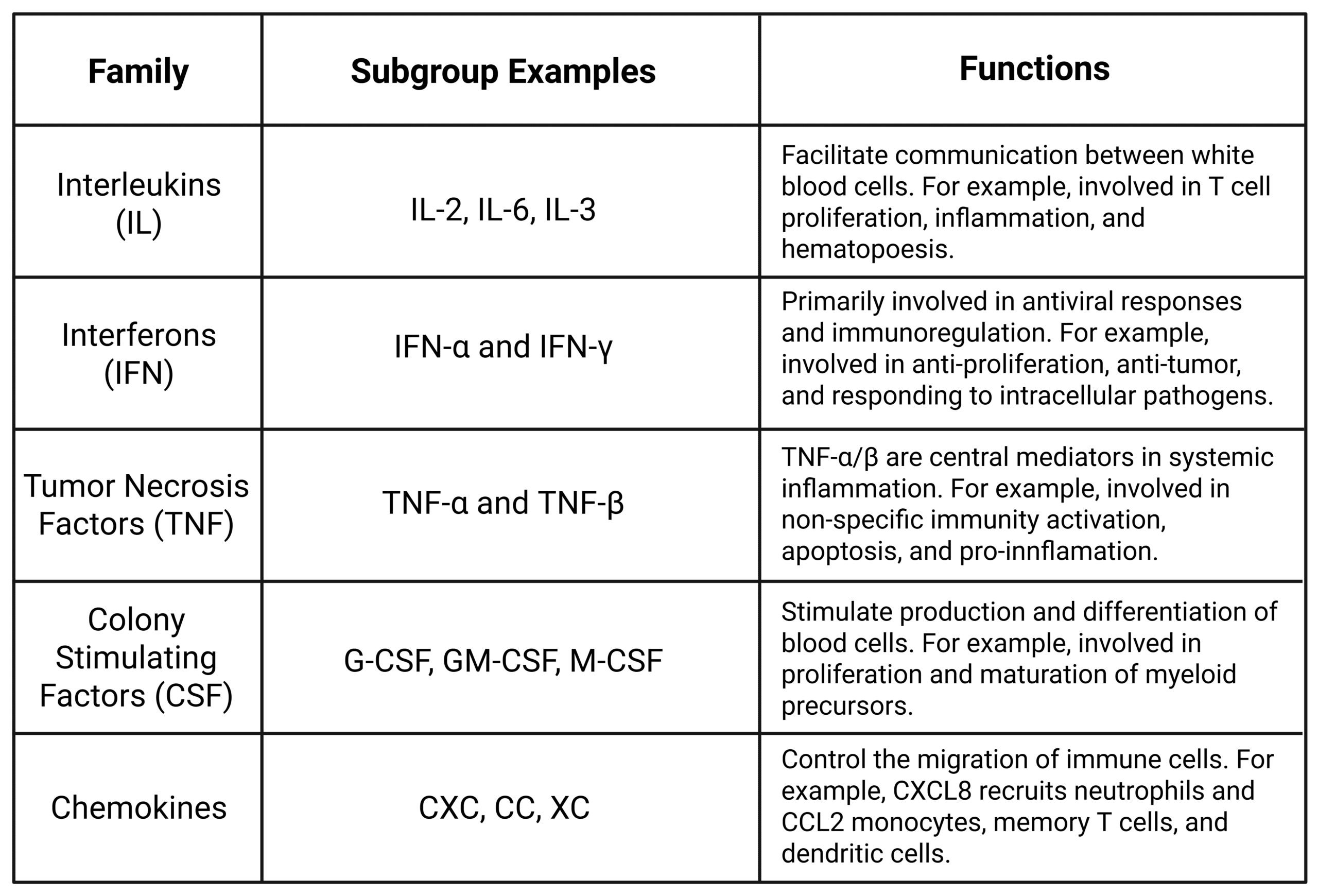
Cytokines are small soluble proteins that act as signaling molecules in the immune system. Interleukins (ILs), interferons (IFNs), tumor necrosis factors (TNFs), and colony-stimulating factors (CSFs) are just a few of the major families of cytokines (Table 1). More recently, cytokines are also classified by how they affect T cells, namely Th1 cytokines (type 1 cytokines), Th2 cytokines (type 2 cytokines), or regulatory cytokines. Th1 cytokines trigger a strong cellular immune response, while Th2 cytokines promote a strong humoral immune response, and regulatory cytokines promote homeostasis to prevent autoimmunity.
Cytokines are secreted from many different types of cells, such as lymphocytes, macrophages, natural killer (NK) cells, mast cells, and stromal cells. Once released into the surrounding environment, cytokines travel through the extracellular space to bind to specific receptors on the surface of target cells, where they trigger various immune responses. Cytokines can be classified as pro- or anti-inflammatory depending on whether or not they activate or inhibit immune cells.
Table 1: Overview of Cytokine Classifications.
What do Cytokines do?
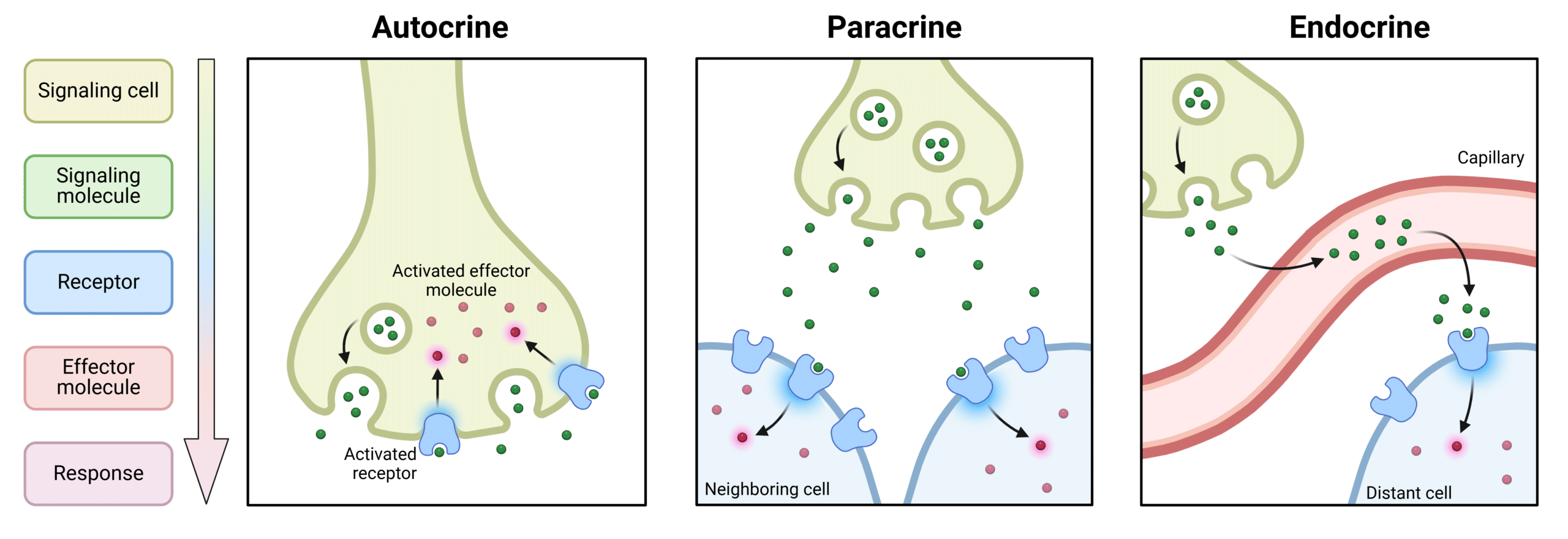
As immunomodulating agents, cytokines have been shown to be involved in autocrine, paracrine, and endocrine signaling (Figure 1). Through these signaling pathways, cytokines are able to regulate cell growth, maturation, and responsiveness of immune cells. A specific type of cytokine can be released from many different types of cells and can produce many different biological activities. As a result, cytokines form an extremely complex network in which different cytokines can have synergistic, antagonistic, and additive relationships to modulate the same biological process. For example, IL-10 inhibits production of pro-inflammatory cytokines.
Figure 1: Types of Signaling Pathways for Cytokines.
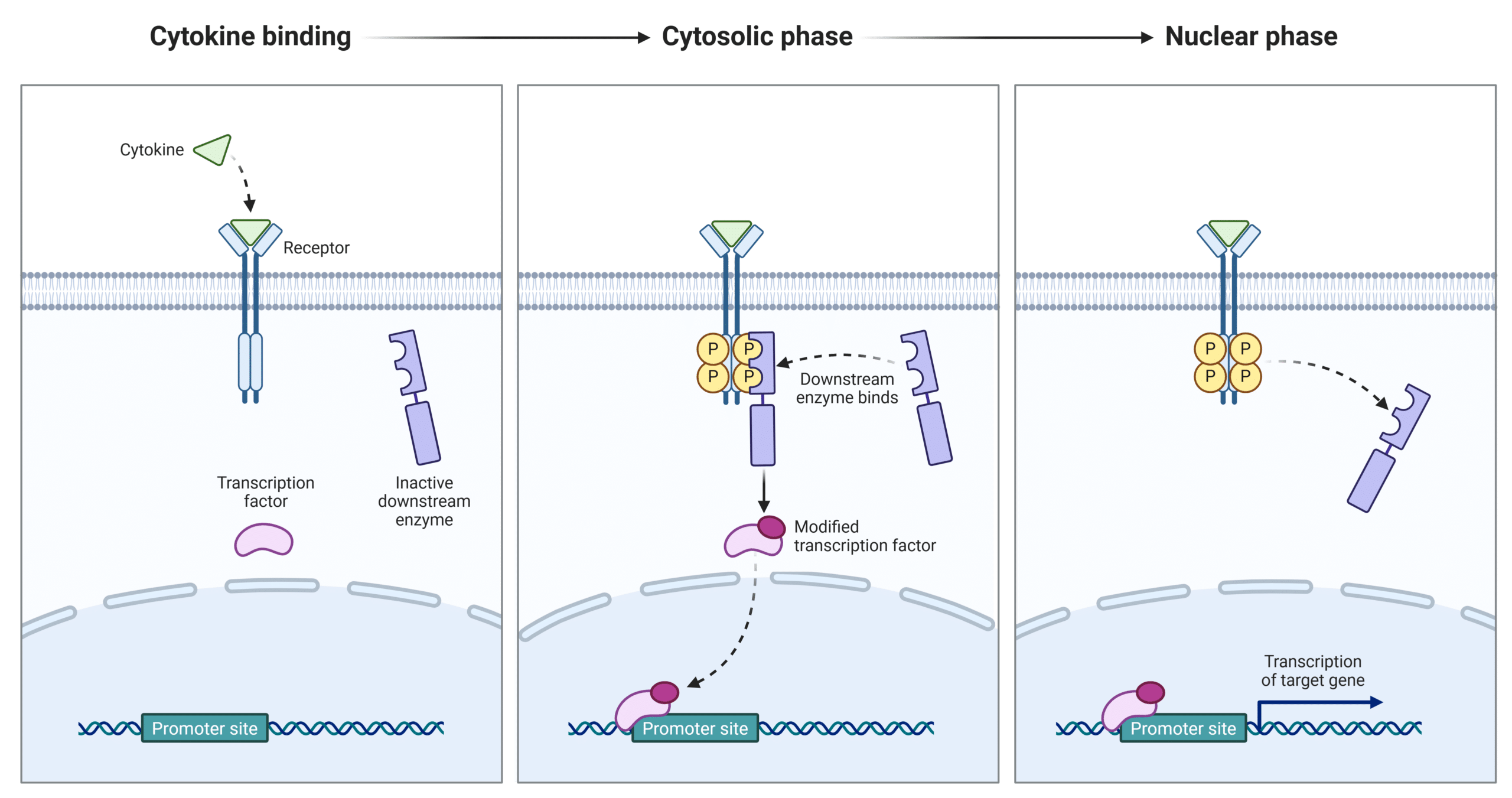
Cytokines exert their effects by binding to specific receptors on the surface of target cells, triggering intracellular signaling pathways such as JAK-STAT, MAPK, and NF-κB (Figure 2). Each cytokine has a matching cell surface receptor and signals changes in gene expression through their transcription factors that regulate immune responses, inflammation, cell growth, and survival. Depending on the cytokine and the context, this can result in release of other types of cytokines, activation of immune cells, recruitment of immune cells to sites of infection or injury, or suppression of immune activity to maintain balance. The response is tightly regulated by feedback mechanisms to prevent overactivation and tissue damage.
Figure 2: General Cytokine Signaling Pathway.
Cytokines in Disease
When we experience injury or infection, cytokines induce an inflammatory response, which involves macrophages releasing pro-inflammatory cytokines such as TNF-α, IL-6, and IL-12. Excessive production of these pro-inflammatory cytokines can lead to chronic inflammation and tissue damage, as seen in autoimmune diseases such as type 1 diabetes, rheumatoid arthritis, inflammatory bowel disease, and multiple sclerosis. For example, chronic expression of IFN-γ has been shown to be associated with autoimmune disease progression.
The example above outlines the importance of anti-inflammatory cytokines such as IL-4, IL-6, IL-10, IL-11, IL-13, and TGF-β. These cytokines inhibit excessive inflammation; for example, IL-10 inhibits the production of many different pro-inflammatory cytokines that play a major role in allergies and asthma. However, just like pro-inflammatory cytokines, excess anti-inflammatory cytokines can be damaging. If anti-inflammatory cytokines overcompensate, this exposes the host to systemic infections.
Our immune system is a double edged sword that needs a delicate balance of cytokines to inhibit any negative health impacts. In infectious diseases, an imbalanced cytokine response can result in a “cytokine storm“, a hyperinflammatory state seen in severe cases of influenza, COVID-19, and sepsis, that can cause organ failure and death. Cytokines are also implicated in cancer, where some promote tumor growth and immune evasion, while others can enhance anti-tumor immunity. Additionally, dysregulated cytokine signaling contributes to allergic conditions, fibrosis, and metabolic disorders. Due to their central role in disease, cytokines are major therapeutic targets, with several drugs developed to block or mimic their actions.
Cytokines in Therapeutics
Cytokines are powerful immune modulators with broad therapeutic potential. Pro- and anti-inflammatory recombinant cytokines have been used to either stimulate immune responses, such as TNF-α for AIDS, or suppress excessive inflammation, such as IFN-β for autoimmunity. However, while their therapeutic potential is significant, systemic administration of cytokines has been limited by challenges such as off-target toxicity, rapid clearance, and the risk of inducing cytokine storms.
To address these limitations, researchers are developing protein engineering strategies that improve the safety, specificity, and efficacy of cytokine therapies. One of the most promising innovations is the use of cytokine fusion proteins to increase half-life, target localization, and introduce functionalities. Of these cytokine fusion proteins that have reached clinical trials, antibody-cytokine fusion proteins, also known as immunocytokines, are among the most promising. These engineered molecules combine the immune activating function of cytokines with the precision targeting ability of monoclonal antibodies. By delivering cytokines directly to diseased tissues, such as tumors or inflamed joints, immunocytokines minimize systemic exposure while amplifying therapeutic impact.
For example, NHS-IL12 combines cytokine IL-12 with an antibody (NHS76) that targets DNA in necrotic tumor areas, helping concentrate the therapy at the tumor site. This approach addresses IL-12’s limitations, poor pharmacokinetics and toxicity, by boosting immune activation (via NK and CD8+ T cells) while minimizing side effects. NHS-IL12 has shown promising tumor suppression in preclinical models and is now being tested in combination with other therapies for treating solid tumors.
Immunocytokine Engineering and Anti-Cytokine Antibody Sequencing with Rapid Novor
Cytokine therapeutics, like immunocytokines, have many challenges during development given their complex nature. Rapid Novor’s de novo protein sequencing technology can help inform protein engineering of antibody-cytokine fusion protein therapeutics.
REmAb de novo antibody sequencing also provides precise amino acid sequences for anti-cytokine antibodies. Whether it’s neutralizing antibodies targeting IL-6, TNF-α, IFN-γ, or others, we provide sequence data with 100% accuracy to support recombinant production, patent filing, biosimilar development, and detailed characterization.
To learn more about Rapid Novor’s de novo antibody sequencing services, contact our scientists!
Talk to Our Scientists.
We Have Sequenced 10,000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and developed the first recombinant polyclonal antibody diagnostics.
Talk to Our Scientists.
We Have Sequenced 9000+ Antibodies and We Are Eager to Help You.
Through next generation protein sequencing, Rapid Novor enables timely and reliable discovery and development of novel reagents, diagnostics, and therapeutics. Thanks to our Next Generation Protein Sequencing and antibody discovery services, researchers have furthered thousands of projects, patented antibody therapeutics, and ran the first recombinant polyclonal antibody diagnostics